



















































































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Thermodynamics and chemical kinetics
Typology: Slides
1 / 140

This page cannot be seen from the preview
Don't miss anything!





























































































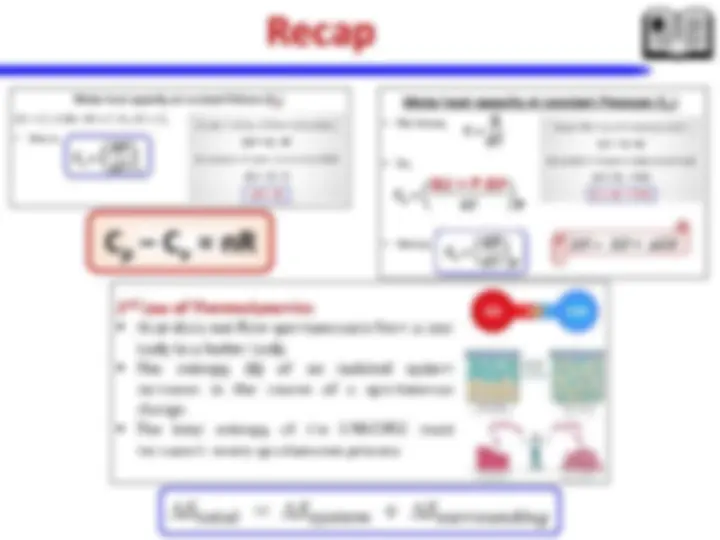
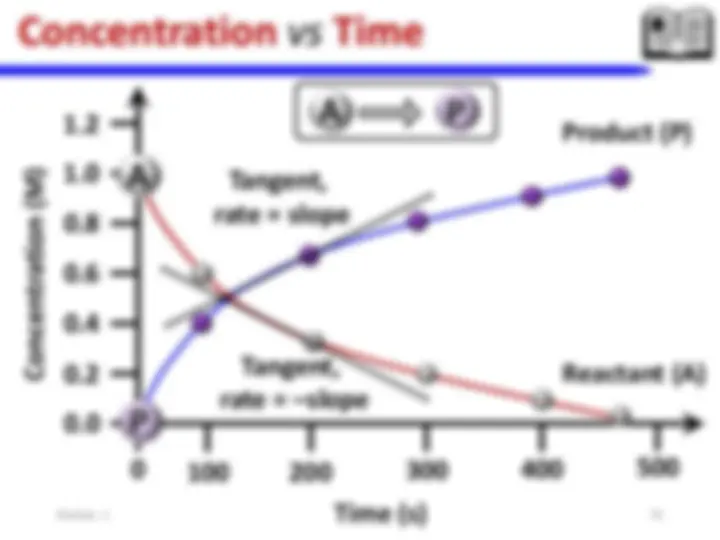
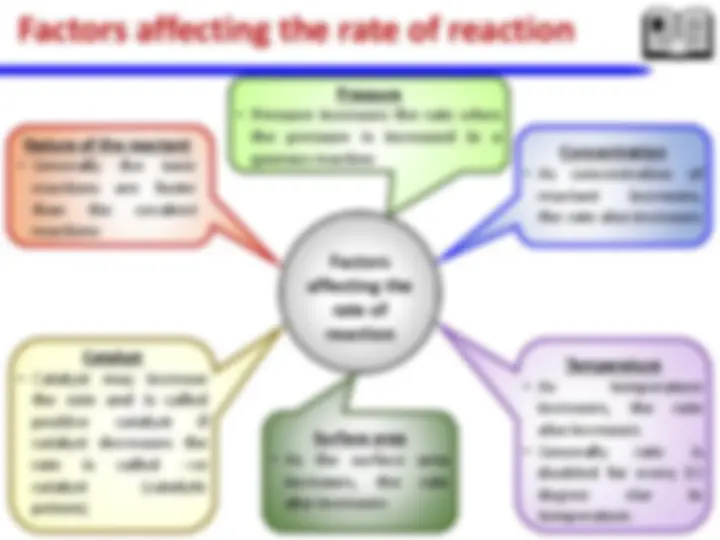
Chemical Thermodynamics: laws of thermodynamics – entropy change (selected processes) – spontaneity of a chemical reaction and Gibbs energy- heat transfer. Chemical kinetics: Concept of activation energy, energy barrier- Arrhenius equation – effect of catalysts (homo and heterogeneous) – Enzyme catalysis (Michaelis-Menten Mechanism).
Chemical Thermodynamics Thermodynamics:
Chemical Thermodynamics Chemical Thermodynamics:
i. System i. System ➢ Part of the universe which under investigation
i. System State of a system
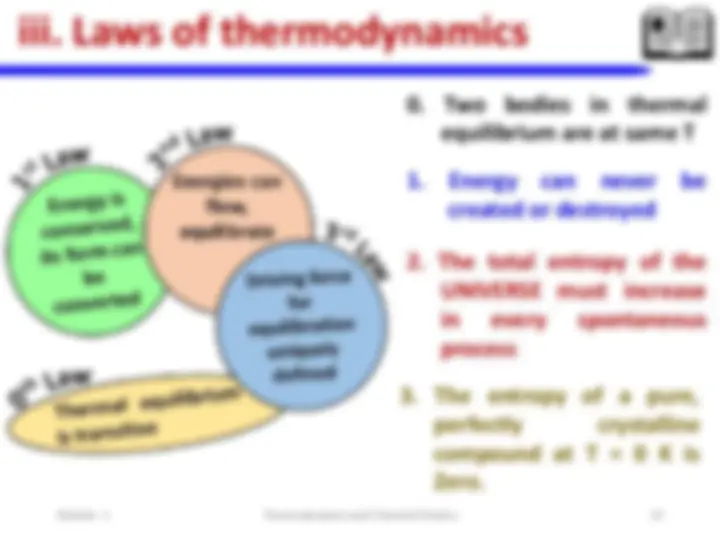
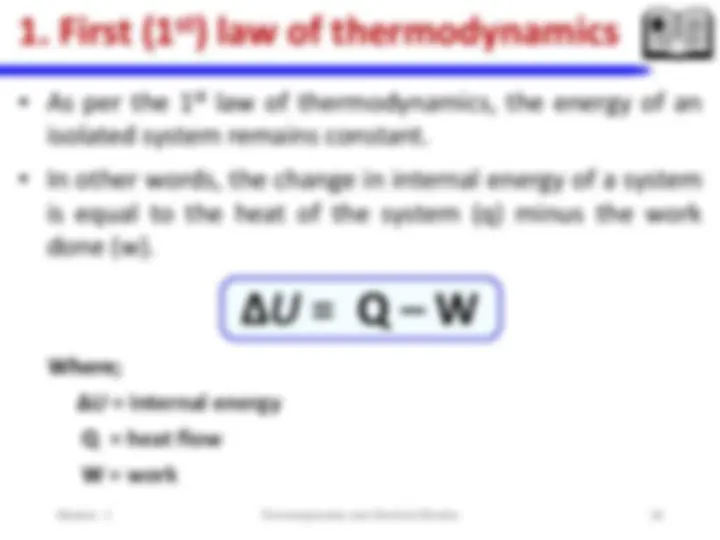
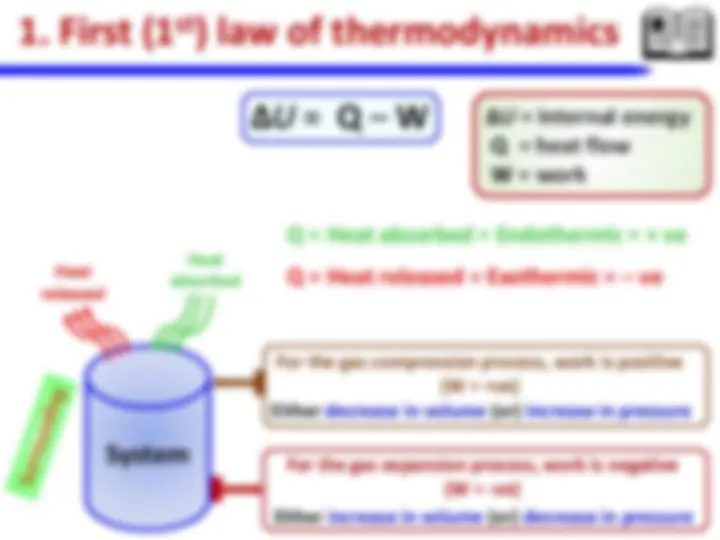
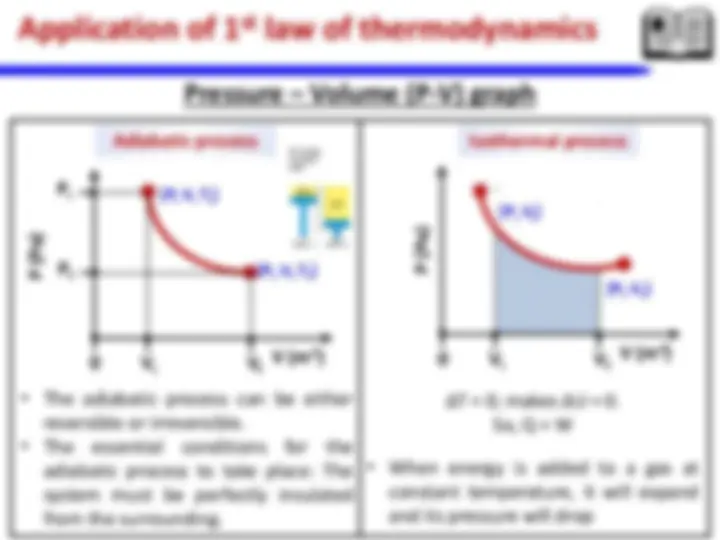
**iii. Laws of thermodynamics
0. Zeroth ( th ) law of thermodynamics
th
c
0. Zeroth ( th ) law of thermodynamics “If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with one another” A B T A
B T c
A
B
C T B
A After a while
Chemical Thermodynamics: laws of thermodynamics – entropy change (selected processes) – spontaneity of a chemical reaction and Gibbs energy- heat transfer. Chemical kinetics: Concept of activation energy, energy barrier- Arrhenius equation – effect of catalysts (homo and heterogeneous) – Enzyme catalysis (Michaelis-Menten Mechanism).
Chemical Thermodynamics: laws of thermodynamics – entropy change (selected processes) – spontaneity of a chemical reaction and Gibbs energy- heat transfer. Chemical kinetics: Concept of activation energy, energy barrier- Arrhenius equation – effect of catalysts (homo and heterogeneous) – Enzyme catalysis (Michaelis-Menten Mechanism).