
















Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Material Science for Engineers which includes Structure of Wood, Moisture Content, Density of Wood, Mechanical Properties of Wood, Expansion and Contraction of Wood, Concrete Materials, Properties of Concrete etc. Key important points are: Thermal Properties of Materials, Heat Capacity, Specific Heat, Thermal Expansion, Thermal Conductivity, Thermal Shock, Function of Temperature, Specific Heat of Tungsten, Casting Process, Protective Coating
Typology: Slides
1 / 24

This page cannot be seen from the preview
Don't miss anything!

















Chapter 21 – Thermal Properties of
Materials
Section 21.
Heat Capacity and Specific Heat
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
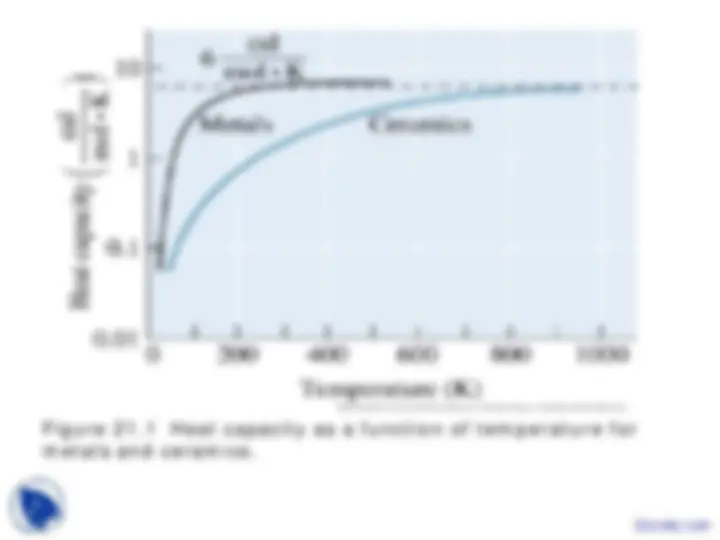
Figure 21.1 Heat capacity as a function of temperature for metals and ceramics.
How much heat must be supplied to 250 g of tungsten to raise its temperature from 25 o^ C to 650 o^ C?
Example 21.1 SOLUTION
Example 21. Specific Heat of Tungsten
If no losses occur, 5000 cal (or 20,920 J) must be supplied to the tungsten.
Suppose the temperature of 50 g of niobium increases 75o^ C when heated for a period. Estimate the specific heat and determine the heat in calories required.
Example 21.2 SOLUTION
The atomic weight of niobium is 92.91 g/mol. We can use Equation 21-3 to estimate the heat required to raise the temperature of one gram by one o^ C:
Example 21. Specific Heat of Niobium
Thus the total heat required is:
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license. Figure 21.3 The relationship between the linear coefficient of thermal expansion and the melting temperature in metals at 25°C. Higher melting point metals tend to expand to a lesser degree.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
Figure 21.4 (a) The linear coefficient of thermal expansion of iron changes abruptly at temperatures where an allotropic transformation occurs. (b) The expansion of Invar is very low due to the magnetic properties of the material at low temperatures.
Example 21.3 SOLUTION
Both silicon and tin are covalently bonded. The strong covalent bonds are more difficult to stretch than the metallic bonds (a deeper trough in the energy-separation curve), so these elements have a lower coefficient. Since germanium also is covalently bonded, its thermal expansion should be less than that predicted by Figure 21.3.
Design the dimensions for a pattern that will be used to produce a rectangular-shaped aluminum casting having dimensions at 25 o^ C of 25 cm × 25 cm × 3 cm.
Example 21.4 SOLUTION
The linear coefficient of thermal expansion for aluminum is 25 × 10 -6^ 1/o^ C. The temperature change from the freezing temperature to 25 o^ C is 660 - 25 = 635 o^ C. The change in any dimension is given by:
Example 21. Design of a Pattern for a Casting Process
For the 25-cm dimensions, l (^) f = 25 cm. We wish to find l 0 :
A ceramic enamel is to be applied to a 1020 steel plate. The ceramic has a fracture strength of 4000 psi, a modulus of elasticity of 15 × 10 6 psi, and a coefficient of thermal expansion of 10 × 10 -6^ 1/o^ C. Design the maximum temperature change that can be allowed without cracking the ceramic.
Example 21. Design of a Protective Coating
Example 21.5 SOLUTION
If only the enamel heated (and the steel remained at a constant temperature), the maximum temperature change would be:
However, the steel also expands. Its coefficient of thermal expansion (Table 21-2) is 12 × 10 -6^ 1/o^ C and its modulus of elasticity is 30 × 10 6 psi. The net coefficient of expansion is
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
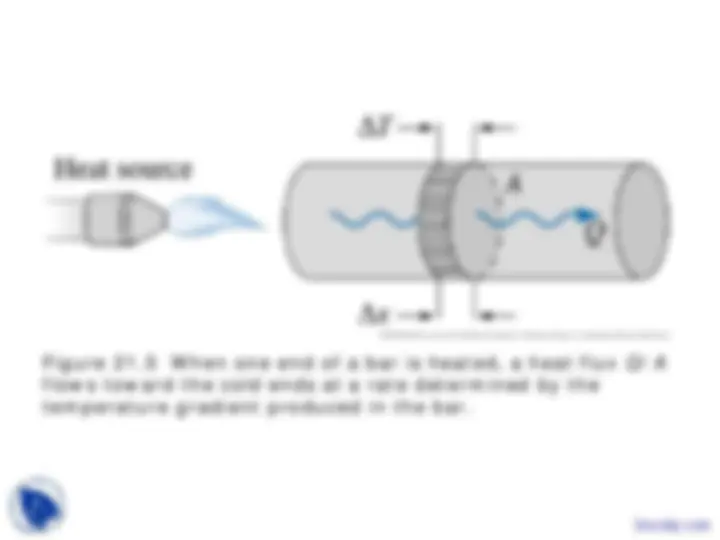
Figure 21.5 When one end of a bar is heated, a heat flux Q/A flows toward the cold ends at a rate determined by the temperature gradient produced in the bar.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
Figure 21.6 The effect of temperature on the thermal conductivity of selected materials. Note the log scale on the y- axis.