














Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
An overview of exercise physiology and training techniques for individuals with spinal cord injuries (SCI) who use wheelchairs. It discusses the importance of exercise for individuals with SCI, the differences in physiologic responses to arm exercise compared to leg exercise for individuals with SCI, and the use of functional electrical stimulation (FES) for exercise of paralyzed lower limb muscles. The document also covers exercise precautions and considerations for individuals with SCI.
What you will learn
Typology: Exercises
1 / 21

This page cannot be seen from the preview
Don't miss anything!














by Roger M. Glaser, PhD; Thomas W.J. Janssen, PhD ; Agaram G. Suryaprasad, MD; Satyendra C. Gupta, MD; and Thomas Mathews, MD
Dr. Glaser is Director of the Institute for Rehabilitation Research and Medicine, Wright State University School of Medicine in Dayton, Ohio and Dr. Janssen is Associate Director. Drs. Suryaprasad, Gupta, and Mathews are Chiefs of Cardiology, Noninvasive Cardiology, and Neurology, respectively at the Dayton VA Medical Center, Dayton, Ohio.
Knowledge of exercise physiology is essential for implementing strategies to develop optimal physical performance among individuals with lower limb paraly- sis due to spinal cord injury (SCI). Since there can be marked neuromuscular changes, it is necessary to take into consideration specific deficits in neuromuscular, cardiovascular, and respiratory function. Indeed, physi- ologic responses to arm exercise performed by individu- als with SCI can be quite different from those for either arm or leg exercise by individuals who are nondisabled, and exercise activities need to be designed to reflect these differences. Individuals with lower limb paralysis due to SCI typically use their arms for wheelchair locomotion and other activities of daily living (ADL), as well as for exercise training and sports activities. However, several physiologic factors, including the relatively small muscle mass that is under voluntary control, deficient cardiovascular reflex responses, as well as inactivity of the skeletal muscle pump of the legs (potentially resulting in a slowing down of circulation), can
This material is based on work supported by the Department of Veterans Affairs, Rehabilitation (^) Research and Development Service, Washington, DC.
markedly reduce the capacity for arm activity (1–3). Muscular weakness and the early onset of fatigue can discourage an active lifestyle, since activities of daily living become relatively more stressful to perform and limit the development of cardiopulmonary (aerobic) fitness. A sedentary lifestyle aggravates this situation, since muscle strength and cardiopulmonary fitness progressively decrease, leading to a debilitative cycle that can be difficult to arrest or reverse (1,2,4). In addition, a number of secondary medical complications that can cause much suffering and greatly increase the cost of medical care, tend to become more prevalent (5,6). However, studies on wheelchair users with SCI indicated that those who maintain a more active lifestyle by regularly participating in exercise and sports pro- grams can increase their muscle strength, cardio- pulmonary fitness, and physical performance to levels well above those of their inactive peers (2,4,7–10). In addition to fitness gains, habitual physical activity may also elicit improvements in health, psychosocial status, rehabilitation potential, functional independence, and quality of life (10-13). Therefore, a major focus of our VA-supported research effort has been to apply exercise physiology principles to develop specialized exercise testing and training techniques for individuals with SCI, and to gain a better understanding of how their
3
RRDS Physical Fitness : A Guide for Individuals with Spinal Cord Injury
muscular, metabolic, and cardiopulmonary responses to various exercise modes differ from those elicited from individuals who are nondisabled. This information may help clarify how physical performance of individuals with paraplegia and quadriplegia can be improved, and how risks for secondary medical complications can be reduced. Arm exercise modes have traditionally been used for the testing and training of wheelchair users. However, physiologic responses to arm exercise per- formed by individuals with SCI can be quite different from those for either arm or leg exercise by nondisabled peers. Our research is related to physical capability and physiologic responses to exercise of wheelchair users with SCI, the use of the arm exercise techniques for physical fitness testing and training, and the use of recently developed training techniques that incorporate functional electrical stimulation (FES)-induced exercise of paralyzed leg muscles. Although most of the subjects who participated in the described research had SCI, many of the techniques and data presented may also be applicable to wheelchair users with other neuromuscular disorders (e.g., head trauma, stroke, multiple sclerosis).
Causes of SCI SCI is a disorder that can cause paraplegia or quadriplegia (tetraplegia) due to lesions that interrupt the transmission of nerve signals (i .e., action potentials) between the brain and periphery. Major causes are motor vehicle accidents (over 38 percent), accidents that occur during sports or physical activities, falls, and trauma during violent crimes (14). It has been estimated that there are more than 200,000 individuals with SCI (48 percent paraplegia, 52 percent quadriplegia) in the United States, and there are approximately 8,000 new cases of SCI each year that survive to join this population (14,15). Prior to World War II, 80 percent of victims with SCI died within 3 years of the injury (16), primarily due to kidney and pulmonary infections (17). However, with the advent of antibiotic drugs and advances in surgical techniques, individuals with paraplegia can have a near normal life expectancy, whereas those with quadriplegia tend to have a life expectancy that is about 10 percent lower than nondisabled individuals (17). Generally, the higher the age at the time of SCI, the higher the lesion level, and the greater the extent of the lesion, the lower will be the life expectancy (18,19). Currently, the prevalent causes of death with long-term SCI appear to be related to a
variety of cardiovascular and respiratory disorders (14,20–22).
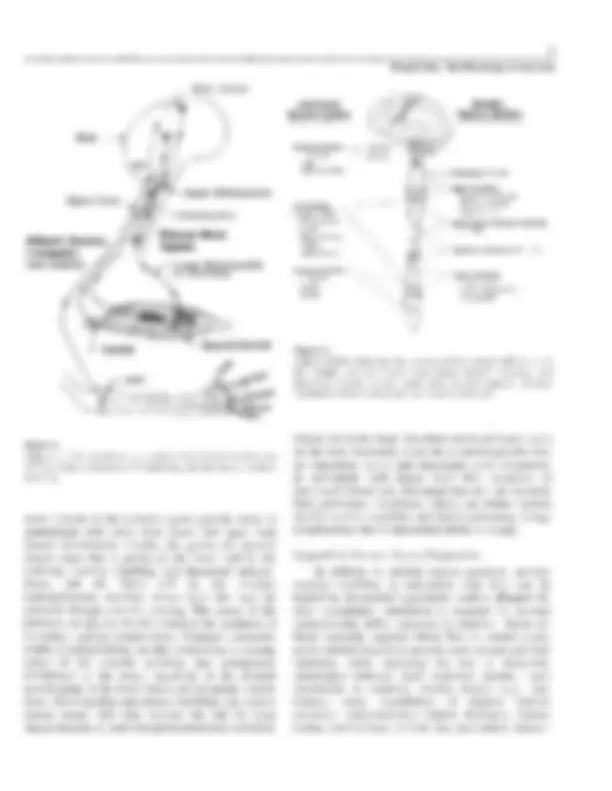
Pathophysiology of SCI and Exercise Limitations This section provides a brief overview of how skeletal muscles are controlled by the central nervous system (CNS ; brain and spinal cord) and how SCI impairs this control and limits exercise capability. Figure 1 is a diagram illustrating efferent (i .e ., motor) and afferent (i .e., sensory) pathways which normally permit precise control of skeletal muscle contractions (1). To initiate skeletal muscle contractions voluntarily, action potentials arise in the motor cortex of the brain and are propagated down the spinal cord along upper motoneurons that eventually synapse onto the lower motoneurons (a motoneurons). Axons of the lower motoneurons leave the CNS and provide motor signals to particular groups of skeletal muscle fibers via neuromuscular junctions. Lower motoneurons and the particular muscle fibers they innervate are called motor units, of which there are about one-half million in the body. Of course, an interruption of the efferent motor pathway would lead to paralysis of the particular skeletal muscle fibers involved. In addition to inducing contractions, the CNS normally monitors performance of the muscles. For this, sensory receptors located in muscles, tendons, and joints (in conjunction with afferent neurons), send feedback information, such as action potentials, to the CNS concerning muscle length, limb position, rate of movement, and contraction tension. This permits stimulation to the muscles to be continuously and appropriately modified so that the actual performance closely matches the intended perfor- mance, and motor learning can take place. Of course, an interruption of this afferent sensory pathway can result in a loss of kinesthetic sense, as well as skin sensations below the lesion level. Figure 2 diagrammatically illustrates the CNS with outflow levels of the somatic nervous system, which innervates skeletal muscles, and the autonomic nervous system, which innervates internal organs (1). The higher the level and more extensive the spinal cord lesion, the more widespread will be the loss of somatic and autonomic nervous system function.
Somatic Nervous System Dysfunction With respect to somatic function, lesions in the thoracic and lumbar regions typically result in paraple- gia with lower limb and partial trunk muscle involve-
RRDS Physical Fitness : A Guide for Individuals with Spinal Cord Injury
tility, stroke volume, and cardiac output (1,2,23–25). Although these reflexes are absent to varying degrees in most individuals with SCI, those with lesions above Ti would have interruption of all sympathetic nerves that innervate the heart (from T1 to T4), which would markedly limit cardioacceleration, myocardial contrac- tility, stroke volume, and cardiac output (26). With this condition, any cardioacceleration that occurs with exer- cise may be primarily due to withdrawal of vagal parasympathetic tone to the S-A node. As a result, persons with complete quadriplegia usually have a peak exercise heart rate (e .g., 100–125 beats/min) that is well below the age-predicted maximal. In addition, the combination of reduced venous return and deficient myocardial contractility decreases the stroke work (i .e ., stroke volume x mean arterial blood pressure) of the heart, which can ultimately lead to loss of left ventricular muscle mass. This is especially prevalent in quadriplegia (27). It is also likely that reduced sympa- thetic outflow with SCI will impair thermoregulatory capacity due to inappropriate blood flow distribution and insufficient sweating response below the lesion level (28).
Exercise Consequences The loss of functional skeletal muscle mass with SCI and inactivity of the skeletal muscle pump in the lower limbs are compounded with diminished or nonexistent cardiovascular reflexes during exercise. This can cause high fatigability of active arm muscles due to their relatively small mass, inadequate blood flow due to hypokinetic circulation, and limited aerobic energy supply, as well as a greater component of anaerobiosis (living in an oxygen-free atmosphere) and the accumulation of metabolites in the muscles (1–3,29). High fatigability of arm muscles during wheelchair locomotion and exercise training can dis- courage many wheelchair users from leading active lives. Unfortunately, a sedentary lifestyle can lead to a further decrement of physical fitness and an even greater reduction of physical capability. Aging further decreases cardiovascular, pulmonary, and muscular function, which can eventually lead to a loss of independence and an increase in medical complications (19). An active lifestyle, which incorporates specific exercise training and/or sports programs, is needed to break this debilitating cycle of sedentary lifestyle/loss of fitness and to enhance one's functional independence and quality of life (1,2,4).
Exercise Precautions and Considerations for Persons with SCI Individuals with SCI who perform strenuous exer- cise are exposed to the usual risks known for nondisabled individuals, as well as additional risks due to their CNS damage and the resulting motor, sensory, and autonomic dysfunction. Generally, exercise guide- lines recommended by the American College of Sports Medicine should be followed (30). In addition, since there can be numerous health risks, it is prudent for wheelchair users to have a thorough medical examina- tion (including an EKG) prior to beginning a strenuous exercise program. This is especially true for older individuals who have been sedentary for many years. Unique risks that should be anticipated for individuals with SCI during exercise include trunk instability, exercise hypotension, orthostatic hypotension, auto- nomic dysreflexia, pressure sores, muscle spasms, and thermoregulatory problems (31-36). Therefore, it is recommended that individuals with SCI, health care professionals, and athletic trainers, be aware of known exercise risks and take appropriate precautions to derive optimal benefits in a safe manner such as: Appropriate Body Support. Where necessary, a security belt should be placed around the individual's upper trunk during arm exercise to prevent malalign- ment and falls due to trunk instability and poor sitting balance. In addition, it is absolutely essential that measures be taken to minimize pressure on weight- bearing tissues to prevent decubitus ulcers. Therefore, it is important to place effective cushions under the ischial tuberosities and other weight-bearing areas, as well as to perform periodic pressure relief (i .e., raising the body off the cushion every 20–30 minutes for 30–60 sec pushups). Blood Pressure Responses. Exercise may elicit blood pressure (BP) responses from individuals with SCI that can be quite different and inconsistent in comparison to those from nondisabled individuals. This is particularly true for persons with high-level SCI who can exhibit a paradoxical drop in blood pressure as exercise progresses (29,34,35,37).^ This so-called exer- cise hypotension is apparently due to a lowering of total peripheral resistance, as blood vessels in active muscles dilate in response to hypoxia and increased concentra- tions of local metabolites (e.g., CO2, lactic acid, heat), without a corresponding increase in cardiac output. As indicated above, cardiac output can be limited by inadequate venous return due to inactivity of the skeletal muscle pump and deficient sympathetically
Chapter One : The Physiology of Exercise
mediated redistribution of blood. Exercise in the upright posture can exacerbate this situation, since it can result in blood pooling in the lower body and orthostatic hypotension due to gravitational effects. The combined effects of exercise hypotension and orthostatic hypoten- sion concurrent with reductions in cardiac output and cerebral blood flow can cause nausea/vomiting, dizzi- ness, or possible loss of consciousness. If symptoms occur either during or following exercise, the individual should be immediately placed in a reclining position to facilitate venous return to elevate cardiac output and blood pressure. Hypotension risk may be reduced by elevating the legs during exercise, regular orthostatic training (e.g., head-up tilt, standing via tilt table, orthotic ambulation), proper hydration, compression stockings, abdominal binder, and physical conditioning. Occasionally, some individuals with high-level SCI may exhibit a sudden and inappropriate episode of extremely high blood pressure (hypertension) due to autonomic dysreflexia (hyperreflexia). This is caused by loss of central control (i .e., inhibition) of spinal reflexes causing exaggeration of some sympathetic responses (increase in noradrenaline) to noxious afferent stimuli such as skin tissue trauma, bladder overdistension, and bowel impaction (31,38). Autonomic dysreflexia can be quite hazardous and possibly lead to fatality if it is not corrected immediately (39–41). To help avoid this condition, it is important that the individual follow proper health practices to eliminate noxious stimuli, and seek medical treatment where appropriate. It is, there- fore, recommended that the bladder be emptied just prior to exercise and during prolonged exercise bouts, and that blood pressure be monitored at regular intervals—at least during initial exercise sessions (32). Of course, if extreme hypertension occurs, exercise should be immediately discontinued, and an upright posture should be maintained until the blood pressure returns to normal. Muscle Spasms. Many individuals with SCI experi- ence occasional spasms in the paralyzed lower limb muscles, ranging from mild to severe in intensity. This is due to a loss of inhibitory drive to motor neurons which makes them hyperexcitable to sensory input. Care must be taken to avoid damage to the lower limbs in the event of strong spasms and rapid limb movements. Pharmacotherapy is often employed to help control for muscle spasms. This treatment may involve the use of oral antispasmodic and muscle relaxant drugs. However, these drugs may further limit exercise capability by not only reducing excitability of the paralyzed muscles, but
also of the non-paralyzed muscles. In addition, there can be detrimental side effects including dizziness, ataxia (irregularity of muscular action), and depression (17). Thermal Stress. Careful consideration should also be given to ambient temperature, relative humidity, type of clothing worn, exercise intensity, and duration in or- der to prevent hyperthermia or hypothermia. Since many individuals with SCI have limited thermoregula- tory capacity due to inadequate secretion by sweat glands and inappropriate distribution of blood due to impaired cardiovascular system control, it is possible that overheating can occur more easily in this popula- tion than for nondisabled individuals (28,42–44). This is especially true in a hot, humid environment where pro- longed strenuous exercise can cause severe dehydration, dangerously elevated body temperature, and possibly heat stroke and circulatory collapse. Under these condi- tions, frequent and adequate fluid replacement is essen- tial. Exercise in cold environments may result in exces- sive heat loss from the body, also exacerbated by im- paired cardiovascular system control. Therefore, if there are symptoms of hyperthermia or hypothermia, exercise should be discontinued, and clothing and environmental conditions should be appropriately adjusted.
Role of Exercise and Wheelchair Sports in SCI Rehabilitation Trauma-induced SCI usually results in sudden and drastic changes in lifestyle where there is a marked decrease in physical activity. It has been found that physical activity in individuals with SCI tended to be lower with higher chronological age and shorter time since injury (45). These variables were also related to a less rewarding life and a decline in psychological adjustments. Sedentary lifestyle can be a major factor leading to consequential degenerative changes in the cardiovascular system (2,46,47). This may help explain the 228 percent higher death rate reported for an experimental group with SCI compared to the age- and gender-matched nondisabled control group in the same study (21). Higher coronary heart disease risk for sedentary individuals with SCI had been indicated by findings of significantly lower blood high-density lipoprotein-cholesterol (HDL-C) concentrations in com- parison to athletes with SCI, as well as sedentary and active nondisabled individuals (46-48). Furthermore, reduced basal metabolic rate due to skeletal muscle wasting and lower daily energy expenditure (49) due to physical inactivity may lead to weight gain. Excessive body weight, which can also contribute to cardiovascu-
Chapter One : (^) The Physiology of Exercise
acceptance. However, these results are most likely influenced by inherent differences of exercise habits, attitudes, beliefs, and personality prior to SCI. It is also apparent that psychosocial outcome of individuals with disabilities is highly influenced by societal attitudes. In this regard, wheelchair sports can have a positive effect upon reducing sti gmatization, stereotyping and discrimi- nation, and acceptance of those with disabilities as fully functioning members of society.
Wheelchairs users are required to employ their relatively small and weak upper body musculature for locomotion and most other activities of daily living. This places them at a marked disadvantage due to the limited maximal power output (PO) capability and peak oxygen uptake for arm exercise, which has been reported to be approximately two-thirds of leg exercise values for nondisabled individuals who are not arm exercise trained (55-58). Arm exercise capability may be further reduced due to factors related to the SCI (as indicated above), as well as diminished muscular and cardiopulmonary fitness resulting from sedentary lifestyle and aging. Furthermore, studies have shown that arm exercise is rather inefficient (energy wasteful), and stressful to the muscles involved, as well as to the cardiovascular and pulmonary systems in comparison to the same intensities of leg exercise (^) (56,59,60). Indeed, compared to walking at the same velocities and le g cycling at the same POs, handrim wheelchair propulsion generally elicited greater magnitudes of physiologic responses (61-63). These differences tended to be more pronounced at the greater exercise intensities that occurred at higher locomotive velocities and when negotiating architectural barriers, such as carpeting and upward grades. When comparing arm crank and wheelchair ergometry to leg cycle ergometry at matched submaximal PO levels in nondisabled subjects, the arm exercise modes elicited greater metabolic stress, as indicated by the higher oxygen uptake and blood lactate (LA) values ; heavier cardiac load, as indicated by higher heart rate (HR), peripheral vascular resistance, intra-arterial blood pressure, and stroke work ; and greater demand on the pulmonary system, as indicated by higher pulmonary ventilation. Arm exercise also tends to elicit lower cardiac output (Q) and ventricular
stroke volume (SV) (56,59,64 67). Lower cardiac output and stroke volume may be due to a greater afterload on the heart because of the higher peripheral vascular resistance, and a lower end diastolic volume due to attenuated venous return of blood to the heart. During arm exercise by individuals with SCI, it is feasible that venous return is restricted by inactivity of the skeletal muscle pump in the paralyzed legs (1,2,23). Furthermore, elevated intrathoracic pressure during handrim stroking might decrease thoracic pump effec- tiveness. These combined factors may reduce the effective blood volume during wheelchair activity and limit maximal PO and peak oxygen uptake. Therefore, wheelchair locomotion, even at low PO levels, can represent relatively high exercise loads that can lead to the rapid onset of fatigue. Excessive cardiovascular and pulmonary stresses that may be elicited can hinder rehabilitative efforts and impose risks upon certain patients, such as those with cardiovascular or pulmonary impairments and the elderly (1,2). As indicated above, individuals with higher level and more extensive spinal cord lesions will be placed at greater disadvantage when performing given activities. This can be especially obvious during sporting events where competitors have a wide range of physical capabilities. Therefore, classi- fication systems based upon anatomical, physiological, and functional considerations have been devised for wheelchair sports competitors in an attempt to better group them according to physical capability, and to improve the fairness of competition.
Limiting Factors (^) for Leg Versus Arm Exercise Exercise stress testing is important since it enables exercise capacity and metabolic and cardiopulmonary responses to be ascertained. By repeating this testing periodically, fitness changes over a period of time can be objectively tracked. To assess physiologic responses to exercise in nondisabled individuals, leg exercise modes such as treadmill walking/running, bench step- ping, and leg cycle ergometry are typically used. Here, a large muscle mass is rhythmically contracted, which can stimulate maximal metabolic, cardiovascular, and pul- monary responses for valid functional evaluation of these systems. Many exercise physiologists suggest that the primary factor limiting maximal PO and oxygen uptake during these tests is central circulatory in nature,
ess : A Guide for Individuals with Spinal Cord Injury
Figure 3.
Figure 4.
11 as making activities of daily living 50), may be counteracted by increased Thus, there is evidence to support that ercise training by individuals with SCI sir health status and reduce cardiovascu- manner as leg exercise training bled individuals (23,48). In in exercise and wheelchair sports ave a profound impact on rehabilitation practices can challenge individuals with le physical obstacles and expand func- Lence. Indeed, sports competition pro- )ortunities for the pursuit of excellence to the Olympic levels depending upon zeds and abilities of the person. Figures ,trate well-trained wheelchair athletes Wheelchair road racers in action. road racing and basketball, respectively. one's motivation to begin exercise rrticipation in wheelchair sports, clini- )nsider prescribing such programs early matic rehabilitation process to optimize
of age, gender, and medical history, it users can derive benefits from appro- ed exercise programs. Well-established 'specificity" and "overload" should be )tain the desired results in an efficient Thus, specific exercise regimens (i .e., otocols) are used for muscle strength nce training, as well as for cardio- ., aerobic) training. It has been clearly lat exercise training has resulted in 1rovements in fitness, which can lead to 1 capability and functional independence, relative stresses for performing given ally living (1,2,4,50—54). There is also appropriate exercise training can poten- ;he risk for occurrence of secondary ications associated with wheelchair con- edentary lifestyles. These include muscle porosis, decubitus ulcers, and a host of ry disorders. e and sports participation can enhance less, as well as societal interactions for °rs, it seems reasonable to assume that be psychological benefits in terms of id body image. Indeed, several studies l that wheelchair users who engaged in reation programs experienced significant kill performance, self-concept and self- Wheelchair basketball players.
ng of exercise it would be tat provides a ocity.
eelchair mounted being collected.
RRDS Physical Fitness : A Guide for Individuals with Spinal Cord Injury
work load are accomplished by increasing the roller resistance created by the ergometer's electronic brake and/or by increasing the speed at which the individual pushes the wheelchair wheel (Figure 8) ; thus simulating the physical stress normally experienced during daily wheelchair propulsion. Moreover, this unique design makes the WAFT appropriate for graded exercise testing and conditioning of persons with widely varying levels of cardiorespiratory fitness. The WAI=T is interfaced to an IBM-compatible computer, which controls the ergometer's roller resis- tance. A computer monitor displays graphical feedback to the user, indicating right and left wheel speeds, target wheel speed, resistance setting, accumulated distance, exercise time, and expended kilocalories (Figure 9). Information provided by the WAFT from graded wheelchair exercise tests is useful for developing
exercise prescriptions, evaluating the effectiveness of conditioning or rehabilitation programs, charting the effects of disability on functional capacity, formulating procedures for predicting peak exercise capacity from submaximal graded wheelchair exercise testing, and conducting exercise physiology research. Exercise stress testing is a well-established tech- nique for the detection of ischemic heart disease. When exercise electrocardiography (ECG) testing is combined with echocardiographic analysis of myocardial contrac- tility, the accuracy of the stress test is improved (81,82). Clinicians and researchers often address treadmill or bicycle ergometry for exercise testing as those suitable for individuals who are not lower limb disabled (i .e., spinal cord injury or amputation). The detection and assessment of coronary artery disease in individuals with lower limb disability is a commonly encountered
Figure 7. WAFT with wheelchair alone.
Chapter One : The Physiology of Exercise
problem. These individuals are unable to satisfactorily complete traditional forms of exercise testing. Many people in this population depend daily on the manual wheelchair for mobility. Therefore, the concept of specificity of exercise suggests that wheelchair exercise would be the desired mode of testing for individuals with lower limb disabilities'. Another wheelchair exercise mode that can be useful for stress testing and training is operating a wheelchair on a motor-driven treadmill (83-86). Figure (^10) illustrates a subject with paraplegia performing an exercise stress test with his own wheelchair on a specially designed and constructed motor-driven tread- mill. Exercise intensity can be regulated by adjustment of velocity, grade (i.e., elevation angle), or by applying additional resistive force via a cable-pulley system (86). Using this exercise mode, Janssen, et al. (52) had groups of individuals with lesions between C4-C8, T1-T5, T6-T10, and T1l-L5 perform a maximal effort
Figure 8. WAFT in use.
For further information on the WAFT, contact W. Edwin Langbein, PhD, Research Physiologist, at Edward Hines Jr., VA Hospital, Hines. IL 60141.
stress test. Generally, the higher the lesion level, the lower were the values for these variables, as indicated previously. This type of exercise system enables better simulation of actual wheelchair locomotion than wheel- chair ergometers, but is not practical for most wheel- chair sports participants. For testing outside the labora- tory environment, a rather simple and inexpensive technique that can be used by most wheelchair users is to propel their own wheelchair at paced or self-selected velocities over an established test course in a standard- ized, repeatable manner (62,63,87). Knowing the length of the course and the time to complete the given task can provide information concerning locomotive perfor- mance capability. Heart rate monitoring may be used to give an indication of relative stress for the exercise bout if cardiovascular reflexes are sufficiently intact.
Stress Testing Protocols The fundamental principles followed for lower body stress testing of nondisabled individuals may be employed for upper body stress testing of wheelchair users. These tests are usually progressive with respect to exercise intensity, and have well-defined submaximal or maximal effort end-point criteria. Protocol design may utilize either continuous (nonstop exercise) or discon- tinuous (alternating exercise and recovery periods) exercise. Discontinuous, submaximal protocols may be preferable for stress testing of wheelchair users, since they are relatively safe and comfortable, and easy to administer. A suitable protocol would be to have exercise bouts that are d 6 minutes in duration, separated by 5—10 minutes of rest. For wheelchair ergometer and arm crank ergometry exercise, the propulsion velocity is typically held constant (e .g., wheel velocity of 3 km•h -' and crank rate of 50 revolutions per minute, respectively) while the braking force (resistance) is incrementally increased to elevate PO level. With wheelchair ergometry, 5 W appears to be an appropriate initial PO, as this level is frequently encountered during daily wheelchair locomotion (89). PO progression increments of 5—10 W may be usable for many individuals, and PO can be limited to (^) 25— W for submaximal tests (73,89,90). (^) For more fit individuals, the PO increment and maximal PO permit- ted can be greater. With arm crank ergometry, the protocol can be the same, but the PO levels used could be up to two times that for wheelchair ergometry. Steady rate physiologic responses can be determined during the last minute of each exercise bout. Criteria for exercise stress test termination include : 1) voluntary
Chapter One : The Physiology of Exercise
performed at a lower percentage of maximal PO, peak oxygen uptake and pulmonary ventilation, and heart rate reserve.
It had been stated that normal daily wheelchair activity may not provide sufficient exercise to train the muscular and cardiopulmonary systems, and it was indicated that supplemental exercise training is neces- sary to stimulate fitness improvement (32,85,95,96). Enhancing exercise capability and cardiopulmonary fitness with specific exercise training programs could increase organ system reserve and make activities of daily living less stressful since they would be performed at lower percentages of maximal PO, as well as of peak oxygen uptake, pulmonary ventilation and heart rate reserve (4,50,52). This could possibly contribute to improved functional independence and rehabilitation outcome. Indeed, with wheelchair locomotion at 7 watts (W), well-trained wheelchair athletes (mean age=25 yr) are estimated to utilize less than 7 percent of their maximal PO and 18 percent of peak oxygen uptake. This is in contrast to 9 percent of maximal PO and 29 percent of peak oxygen uptake for their sedentary colleagues. Older, sedentary wheelchair users have a more difficult plight in that 50—60-yr olds may utilize 44 percent of maximal PO and 51 percent of peak oxygen uptake, whereas 80–90-yr olds may be required to use 100 percent of their maximal PO and peak oxygen uptake for this routine locomotive task (63,90). In a 3-year study, Janssen, et al. (50) demonstrated that a decline of only 5—10 W in PO capability of sedentary wheelchair users with quadriplegia could result in a loss of independence. In contrast, however, their peers who regularly participated in sports activities and increased physical fitness generally maintained their independence and performed activities of daily living with less stress. Thus, it is feasible that regular exercise may reduce the stresses of wheelchair locomotion and other activities of daily living, retard the decline in physical capability that typically accompanies aging, and lower some of the risks associated with secondary cardiovascular disabili- ties.
Arm Exercise Training Protocols To enhance muscular and cardiopulmonary fit- ness, as well as performance, arm exercise training for
wheelchair users should, like leg exercise training, incorporate the fundamental principle of overload (1,2,97). Thus, exercise should be performed at inten- sities and/or durations that are beyond those normally encountered during daily activities. Furthermore, exer- cise intensity and/or duration should be progressively increased as performance improves until fitness goals are reached. Regular exercise at the final intensity/ duration levels is required to maintain the achieved fitness status. If exercise is discontinued, detraining will occur and fitness level will diminish in a matter of several weeks (98). For optimal outcome, exercise should also follow the principle of exercise specificity where the mode used should be closely matched to the activity in which performance improvement is desired. Thus, the biomechanics involved and the physiologic responses elicited would be more representative to produce the desired gains in performance. Arm exercise protocols may be either continuous or discontinuous (intermittent) in design. If enhancing cardiopulmonary fitness is the primary goal, PO should be adjusted to moderate levels that enable exercise bouts of relatively long durations (e .g., 15–60 minutes for continuous bouts, and 3–5 minutes for each of the several discontinuous bouts) without eliciting excessive fatigue or respiratory distress (i .e., marked accumulation of lactate in the blood). Exercise sessions should occur 2–5 times per week (97). Traditionally, arm crank ergometry exercise has been used for endurance training of wheelchair users. This exercise mode is readily available and it has been shown to improve cardiopulmonary fitness (53,54). (^) Although wheelchair ergometer exercise elicits similar peak metabolic and cardiopulmonary responses (77,79,92), it has the advan- tage of more closely resembling actual wheelchair activity so it may better enhance locomotive perfor- mance. In contrast to aerobic training, if enhancing muscular power is the primary goal, higher levels of PO would be used and exercise bouts would be of relatively short duration (e .g ., from a few seconds to a few minutes). The large anaerobic energy component would result in a marked accumulation of lactate in the blood. This form of exercise would be useful for wheelchair athletes who want to improve sprinting performance, as well as for most other wheelchair users, since many activities of daily living (e .g., transfers, overcoming architectural barriers) require intense, short duration efforts. When developing an aerobic training program for leg exercise by nondisabled individuals, a suitable
RRDS Physical Fitness: A Guide for Individuals with Spinal Cord Injury
intensity can be established by maintaining heart rate between 60–90 percent of their heart rate reserve, which usually corresponds to 50–85 percent of peak oxygen uptake. The objective is to exercise at high enough intensity for a sufficient duration to permit cardio- pulmonary adaptations to occur. But, exercise intensity should be kept below the point where marked anaerobiosis occurs and a high concentration of lactate accumulates in the blood, which can severely shorten endurance and create discomfort. Actual measurement of heart rate during maximal effort exercise for each individual would be preferred over prediction tech- niques to facilitate appropriate setting of training intensity. But for arm exercise by individuals with SCI, an effective training intensity may be more difficult to set, since heart rate response can be quite diverse. Thus, actual measurement of maximal heart rate can be important. Janssen, et al. (52) showed that percentage of peak oxygen uptake and heart rate reserve were highly correlated during wheelchair exer- cise on a treadmill by individuals with paraplegia and quadriplegia. However, the absolute values for heart rate were lower in the individuals with the higher level lesions. Their regression equations predicted that exer- cise at 50–85 percent of peak oxygen uptake corre- sponded to heart rate reserve values of 40–85 per- cent for individuals with low-level paraplegia and 30–80 percent for those with high-level paraplegia and quadriplegia. Thus, heart rate response expressed either as a percentage of heart rate reserve or max- imal heart rate can be a usable indicator of exercise stress in these populations, but the actual value used would be dependent upon the exercise response charac- teristics of the individual (52,99). Therefore, to set arm exercise intensity for various individuals with SCI, direct determination of peak values for PO, oxy- gen uptake, pulmonary ventilation, heart rate, and blood lactate concentration (during stress testing) would be desirable if the instrumentation is available. In individuals who do not exhibit a clear relationship among these variables (which is more common in those with quadriplegia), training at a percentage of maximal PO may be preferable (100). Where laboratory testing is not available, intensity criteria may be established according to the subjective feeling of stress and the actual exercise endurance capability. In most cases, it is likely that several trials will be needed for each individual to effectively set exercise intensity.
Physiologic Adaptations to Arm Exercise Training Studies on individuals with lower limb disabilities indicated that several weeks of endurance-type arm exercise training can significantly increase PO capabil- ity, peak oxygen uptake, and cardiopulmonary perfor- mance (32,91,101). Arm crank ergometry training of active wheelchair users increased their peak oxygen uptake by 12–19 percent in 7–20 weeks (91). Even greater gains in cardiopulmonary fitness were obtained in only 5 weeks of training for individuals with quadriplegia who had relatively low initial fitness levels (101). Using wheelchair ergometer exercise, Miles, et al. (102) reported that 6 weeks (3 times per week) of interval training by 8 wheelchair athletes resulted in increases of 31 percent for maximal PO capability, 26 percent for peak oxygen uptake, and a 32 percent for peak pulmonary ventilation. These gains were even more remarkable considering that the athletic subjects used had relatively high levels of fitness prior to participating in the program. Although arm exercise limits the absolute level of aerobic fitness that can be achieved with training some cardiopulmonary benefits can be expected for most participants. However, the magnitudes by which aerobic fitness and exercise performance can be increased with training appear to depend on the initial fitness level and the size of the muscle mass available for exercise. For instance, several studies on wheelchair athletes perfonni- ing maximal effort arm crank ergometry and wheelchair ergometer exercise indicated that their peak oxygen uptake is in the 2–3 L/minute range (1,102–105). This is approximately one-half of the maximal oxygen uptake that would be expected for healthy nondisabled athletes performing maximal effort leg exercise (e .g., cycling, running). Greater gains in fitness may be expected from individuals with SCI who initiate training programs at relatively low fitness levels depending upon pathologi- cal limitations (1,2). It is plausible that many of the observed gains in arm exercise performance are due to peripheral adaptations such as hypertrophy, enhanced arterial vasodilation, and improved capillary density and/or metabolic capability within muscles (which
central circulatory adaptations (106). Yet habitual arm exercise training appears to increase maximal PO capability and peak oxygen uptake, and may also decrease levels of physiologic responses for given submaximal exercise tasks and ADLs, including wheel- chair locomotion (107,108).
RRDS Physical Fitness: A Guide for Individuals with Spinal Cord Injury
anti-G suit, but fluctuated pressure every 2 minutes to simulate skeletal muscle pump activity, and found a significant increase in peak oxygen uptake during arm crank ergometry and wheelchair ergometry exercise by individuals with predominantly high-level SCI. Al- though use of external compression to the lower limbs and abdomen may have some effect on facilitating venous return during arm exercise, use of rhythmic contractions of lower limb muscles that are induced by a multichannel electrical stimulator may better activate the skeletal muscle pump for more effective results.
Functional electrical stimulation (FES) research has been conducted for almost 20 years with the goal of inducing exercise in paralyzed lower limb muscles (116). Several devices are now commercially available, so FES-induced exercise can be used by those who are interested in expanding their training capability. This technique typically uses electrical impulses (from a stimulator), which are applied to muscle motor points via skin surface electrodes, to directly induce tetanic contractions of controlled intensity. Therefore, FES- induced exercise of the paralyzed legs has the potential of utilizing a large muscle mass that otherwise would be dormant. Furthermore, this exercise can augment the circulation of blood by activation of the skeletal muscle pump. It is thus apparent that FES exercise modes can improve the health, cardiopulmonary fitness, and reha- bilitation potential of individuals with SCI to levels higher than can be attained with only arm exercise. Individuals with quadriplegia would most likely find this induced exercise mode to be particularly advanta- geous due to the small muscle mass that is under their voluntary control.
Considerations and Precautions for FES Exercise The primary requirement for FES use is that the muscles to be exercised are paralyzed due to upper motor neuron damage, and that the motor units (lower motor neurons and the skeletal muscle fibers they innervate) are intact and functional. The presence of stretch reflex activity and spasticity may indicate that the individual can perform FES exercise. But, if the individual retains some degree of feeling in the skin, FES may cause discomfort or pain at the high
stimulation current levels required to induce forceful contractions. Before participating in an FES exercise program, the individual should undergo a medical examination, which includes radiographs of the paralyzed limbs, range of motion testing, neurological examination, and an EKG. He/she should be informed of the potential benefits and risks of FES exercise, and clearly under- stand that FES will not regenerate damaged neurons and cure paralysis. It should also be understood that, similar to voluntary exercise training, any health and fitness benefits derived from FES exercise training will be lost several weeks after this activity is discontinued. FES-induced contractions should be kept as smooth as possible and the contraction force generated should be limited to a safe level to prevent injury, since the muscles, bones, and joints of paralyzed lower limbs tend to be deteriorated. Although FES exercise training can improve the strength and endurance of the para- lyzed muscles, there is currently no evidence that this activity can reverse osteoporosis. Therefore, with con- tinued training, the muscles could ultimately produce more force than the bones can endure. Furthermore, FES may trigger severe muscle spasms, so it is important that the quality of the contractions be observed by a physician to insure that they are not hazardous (117). In individuals with high-level SCI, FES exercise may provoke autonomic dysreflexia (36,116). Blood pressure should, therefore, be moni- tored periodically, especially during initial FES use. Of course, exercise should be discontinued immediately, as indicated above, if any response is observed that places the individual at risk.
FES-Induced Leg Muscle Contractions to Promote Venous Return As indicated above, arm exercise performance, and the ability to develop high levels of cardiopulmonary fitness, may be restricted by hypokinetic circulation due to impaired skeletal muscle pump activity. Although techniques to promote venous return, such as exercising in a supine position and use of external compression devices, may help alleviate this problem, another viable approach to promote venous return and enhance cardiac output and blood flow to the exercising upper body muscles is FES-induced rhythmic contractions of the paralyzed leg muscles. It is feasible that FES may have several clinical applications including deep venous thrombus prophylaxis, reducing excessive edema, and alleviating orthostatic hypotension.
Chapter One : The Physiology of Exercise
FES-Induced Resistance Exercise It has been shown that the same resistance training principles known to be effective for muscle strengthen- ing by nondisabled individuals can be adapted for FES-induced training of paralyzed muscles. These include dynamic contractions through a specific range of motion, progressive overload, and multiple sets of exercise consisting of a relatively low number of repetitions at relatively high load resistance (117,1 18). Research studies clearly indicate that several weeks of FES-induced weight training exercise of the quadriceps muscles can markedly increase their strength and endurance for this induced activity (117-119).
FES-Induced Leg Cycle Ergometer Exercise A leg cycle ergometer (LCE) is propelled by FES-induced contractions of the paralyzed lower limb muscle groups. Computer-controlled FES is used to induce contractions of the quadriceps, hamstring, and gluteal muscle groups during particular angle ranges of the pedals. Thus, pedaling at the 50 revolutions per minute target rate induces a total of 300 muscle contractions per minute. A microprocessor, which receives pedal position and velocity feedback informa- tion from sensors, controls the cyclic stimulation pattern and cun-ent intensity. As muscle fatigue progresses during exercise, FES current increases automatically to a maximum of about 140 mA to recruit additional muscle fibers in an attempt to maintain revolutions per minute. When the pedal rate falls below 35 revolutions per minute, exercise is automatically terminated.
This chapter presents an overview of what is known about exercise physiology as applied to those with SCI. Research has clearly shown that there can be marked differences in physiologic response patterns between individuals with SCI performing arm exercise and nondisabled individuals performing either arm or leg exercise. There can also be marked differences in physiologic response patterns among individuals with SCI as influenced by the level and extent of the lesion. Thus, when establishing guidelines for exercise testing and training techniques, programs would have to be adapted with respect to the performance characteristics of particular individuals to provide optimal efficacy and to minimize risk. Furthermore, FES-induced exercise of the paralyzed lower limb muscles can possibly elicit
superior physiologic responses than for use in only arm exercise, especially for those with quadriplegia. Clearly, individuals with SCI can derive health and fitness benefits, and reduce the relative stresses for performing activities of daily living when habitually participating in appropriately designed exercise programs and sports activities. Conversely, it is also clear that maintaining a sedentary lifestyle can cause losses in health, fitness, and rehabilitation potential. Therefore, knowledge of exercise physiology is important to provide motivation for those with SCI and to help insure successful outcome for participation in exercise and sports pro- grams, which may have a positive impact on the quality of life.
ADL=activities of daily living CNS=central nervous system EKG=electrocardiogram FES=functional electrical stimulation L=lumbar L/min=liters per minute mA=milliamperes SCI=spinal cord injury WAFT=wheelchair aerobic fitness trainer
ACKNOWLEDGMENTS
The authors wish to thank the Dayton Veterans Affairs Medical Center and the Rehabilitation Institute of Ohio at Miami Valley Hospital for allowing the authors to conduct their exercise research projects on wheelchair users. We would also like to thank all participants in this study.
REFERENCES
Glaser RM. Exercise and locomotion for the spinal cord injured. In : Terjung RE, ed. Exercise and sports sciences reviews. New York : MacMillan Publishers. 1985 :13 :263-303.
Chapter One : The Physiology of Exercise
42 Binkhorst RA, Hopman MTE. Heat balance in paraplegic individuals during arm exercise at 10 °C and 35 °C. Med Sci Sports Exerc 1995 :27(5 Suppl) :S83.
RRDS Physic& Fitness : A Guide for Individuals with Spin& Cord Injury
ergometry. J Appl Physiol : Respirat Environ Exerc Physiol 1980 :48 :1060—4.