

Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Calculation of Lattice Constants and Atomic Radii for Aluminum, Exams of Mechanical Engineering
The steps to calculate the lattice constant and atomic radius of aluminum using its density and atomic weight. It also includes a diagram of a simple cubic lattice and the (122) plane and [122] direction.
Typology: Exams
1 / 1

This page cannot be seen from the preview
Don't miss anything!

Related documents
Partial preview of the text
Download Calculation of Lattice Constants and Atomic Radii for Aluminum and more Exams Mechanical Engineering in PDF only on Docsity!
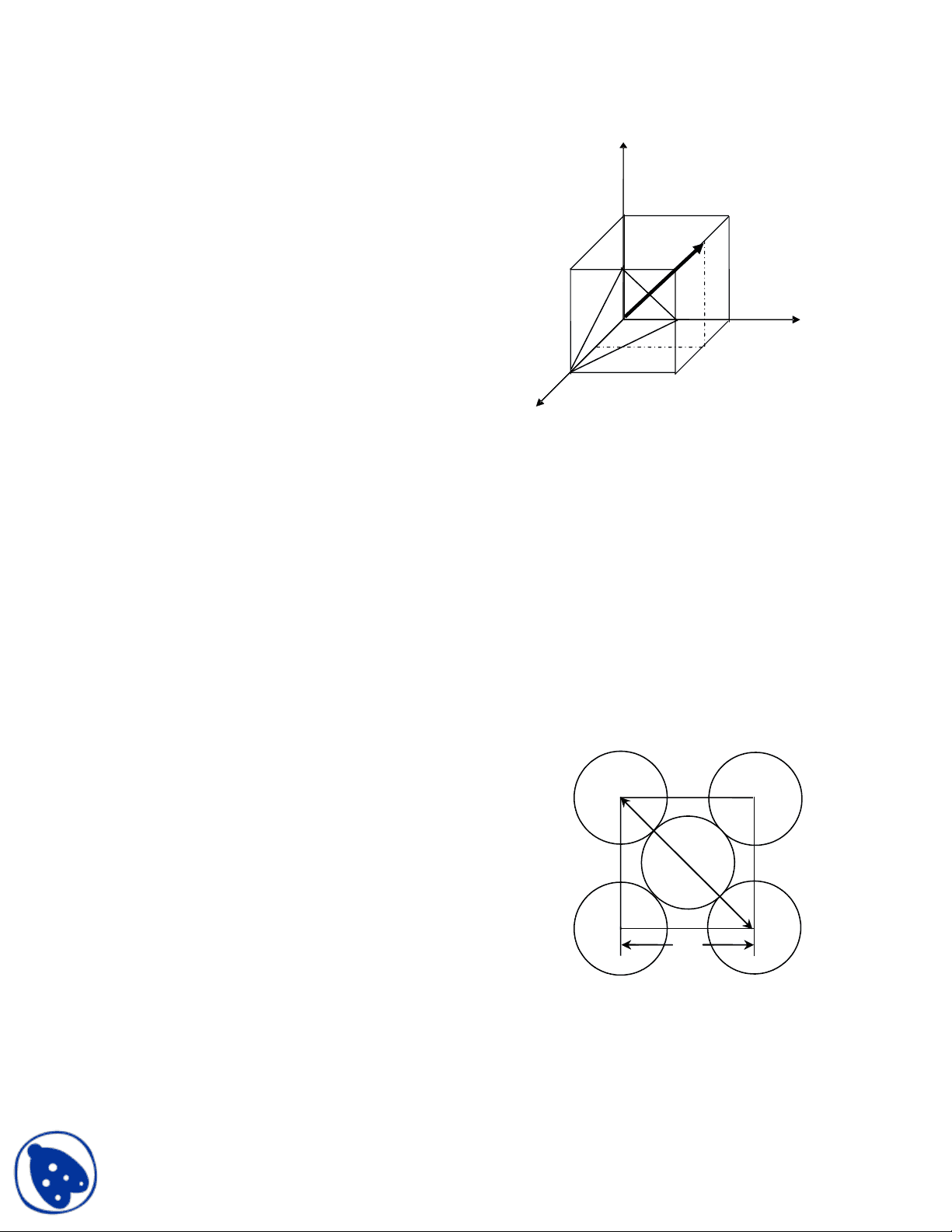
- Draw a space lattice of simple cubic lattice, then
a) Find out the (122) plane and [122] direction
b) Calculate the angle between (122) plane and [122] direction
Answer: (a) See the attached figure for (122) plane and [122]
(b) cos θ = (1×1 + 2×2 +
2 ×2)/ √(1^2 +2^2 +2^2 )^2
then θ = 0o^ (note: people use normal line of a plane to represent the plane)
- The density of aluminum (Al), which has the fcc structure, is 2.70g/cm3. The atomic weight of aluminum is 26.98g/mol. Calculate
a) The lattice constant of aluminum
b) The atomic radium of aluminum
Answer: (a) mass of one Al atom = (26.98 g/mol) / (6.02 × 10 23 atoms/mol)
= 4.48 × 10 -23g/atom
density = (mass of unit cell)/(volume of unite cell) For fcc: density = (mass of 4 atoms)/a^3
Then 2.7 g/cm^3 = 4 × (4.48× 10 -23g)/ a^3
a = 4.05 × 10 -8^ cm
(b) Look at the diagonal line of fcc’s [100] plane,
√2 a = 4 × r
r = √ 2 a/4 = 1.43 × 10 -8^ cm
4r
a

