



Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
It contains objective and assertion-reason type questions with detailed solutions.
Typology: Exercises
1 / 4

This page cannot be seen from the preview
Don't miss anything!



O
PBr Mg CH CH
3 2 2
3
|
3
H O
2
Here, D is [BVP 2004]
(a)
2 3
3
|
3
O CH CH
CH
CH CH
(b)
2 3
3
|
3
CHCH
CH
CH O CH
(c) CHCHOH
CH
CH CH
2 2
3
|
3
(d)
CHOH
CH
CH CH CH
2
3
|
3 2
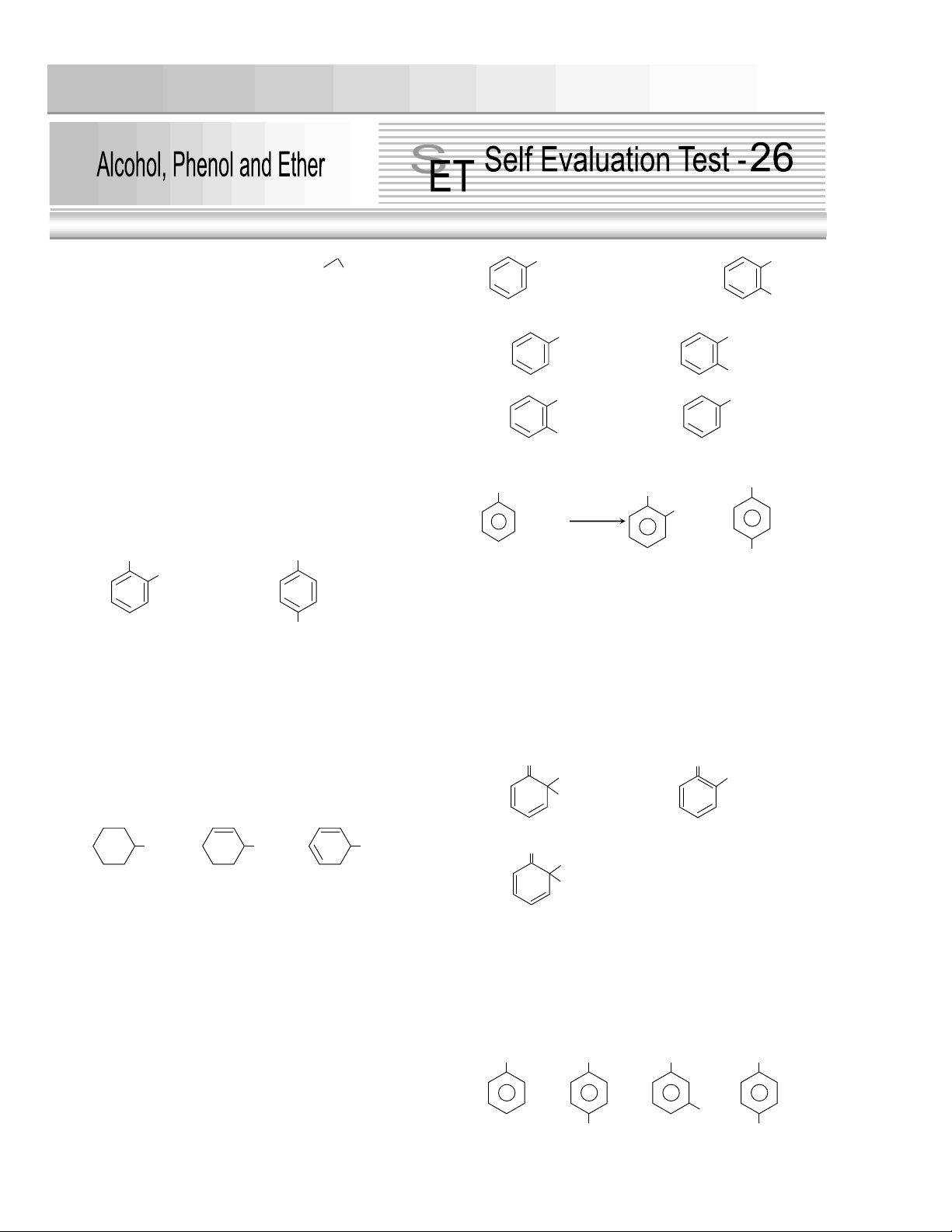
2. Phenol is more acidic than [Pb. CET 2003]
(a) (b)
(c)
2 2
CH (d) Both (a) and (c)
3. In the reaction,
( ) ( )
3
6 5 3 2
C HCHO CHCO O A
CH COONa
product ( A )
is [Pb. CET 2000]
(a) Acetaldehyde (b) Cinnamic acid
(c) -nephthol (d) Phenol
4. The correct order of ease of dehydration of
following is
[CPMT 2004]
(a) I > II > III (b) III > II > I
(c) I > III > II (d) III > I > II
5
PCl reacts with a compound containing [Pb. CET 2002]
(a)
3
SO group (b) – OH group
(c)
3
NO group (d) – NO group
6. Cumene process is the most important
commercial method for the manufacture of
phenol. Cumene is [KCET 2004]
(a) 1 - methyl ethyl benzene (b) Ethyl benzene
(c) Vinyl benzene (d) Propyl benzene
7. The compound X in the reaction [Roorkee 1999]
is
(a) (b)
(c) (d)
8. Reaction
2
2
acidor base
2
is called [MP PET 2003]
(a) Lederer Manasse reaction
(b) Claisen condensation
(c) Benzoin condensation
(d) Etard reaction
9. When phenol is reacted with
3
CHCl and NaOH
followed by acidification, salicyldehyde is
obtained. Which of the following species are
involved in the above mentioned reaction as inter
mediate [DCE 2000]
(a) (b)
(c) (d) All of these
10. The order of solubility of alkanols in water is
(a) Propanol < Butanol > Pentanol
(b) Propanol > Butanol > Pentanol
(c) Propanol > Butanol < Pentanol
(d) Propanol = Butanol = Pentanol
11. In the following compounds
I
II
II
I
a
390 K
HCl
COONa ON
a
COONa
a
a
3
CCl
2
CHCl
OH
CHCl
|
(I
)
3
(I
I)
2
(II
I)
2
(IV
)
The order of acidity is [IIT-JEE 1996]
(a) III IV I II
(b) I IV III II
(c) II I III IV (d) IV III I II
12. Butanal with dilute NaOH gives [UPSEAT 2000]
(a) CHCHCHCHO
OH
H
CH CHCH CCH
2 2 2
|
|
3 2 2 2
(b) CHCHCHO
O
CH CHCH CCH
2 2
||
3 2 2 2
(c) OHCCH CHCHCHCHCHCHO
2 2 2 2 2 2
(d)
3
|
2
|
|
|
3 2 2
13. The correct order of the solubility of the different
alcohols in water is [Pune CET 1998]
(a) n - propyl alcohol > ethyl alcohol > n - butyl
alcohol
(b) Ethyl alcohol > n - butyl alcohol > n - propyl
alcohol
(c) n - butyl alcohol > n - propyl alcohol > ethyl
alcohol
(d) Ethanol > n - propanol > n - butyl alcohol
14. Which one of the following will most readily be
dehydrated in acidic condition [IIT-JEE (Screening) 2000]
(a) (b)
(c) (d)
15. Which of the following compounds will be most
easily attacked by an electrophile [CBSE PMT 1998, 99]
(a) (b)
(c) (d)
16. Fittig's reaction produces
(a) Alkane (b) Alcohol
(c) Diphenyl (d) Diethyl ether
17. p-cresol reacts with chloroform in alkaline
medium to give the compound A which adds
hydrogen cyanide to form, the compound B. The
latter on acidic hydrolysis gives chiral carboxylic
acid. The structure of the carboxylic acid is
[AIEEE 2005]
(a) (b)
(c) (d)
Cl
3
2
3
2
15. (c) Phenol is most easily attacked by an
electrophile because presence of – OH group
increases electron density at o- and p-
positions.
16. (c) 2 C HCl 2 Na CH CH 2 NaCl
diphenyl
6 5 6 5
ether
Dry
6 5
17. (b)
3
3
CHCl 2
OH
CHCl
3
OH
HCN
H O
3