











Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
This presentation is part of Solid State Physics course requirement at Guru Jambheshwar University of Science and Technology. It includes: Real, Crystals, Inversion, Symmetry, Elements, Operations, Plane, Center, Axis, Indistinguishable, Schonflies, Symbol
Typology: Slides
1 / 22

This page cannot be seen from the preview
Don't miss anything!















ELEMENT operation symbol
Symmetry plane reflection through plane= σ
translated to – x,-y,-z
Proper axis =rotataion by 360/n = C
Improper axis 1=rotation by 360/n
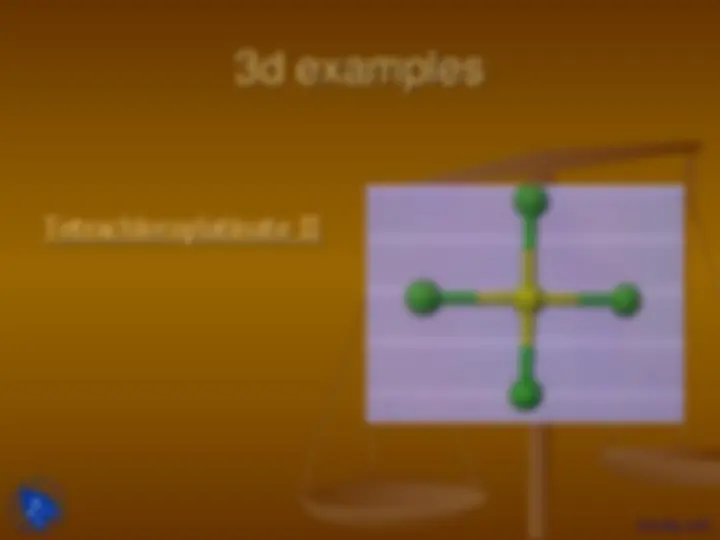
3d examples
Tetrachloroplatinate II
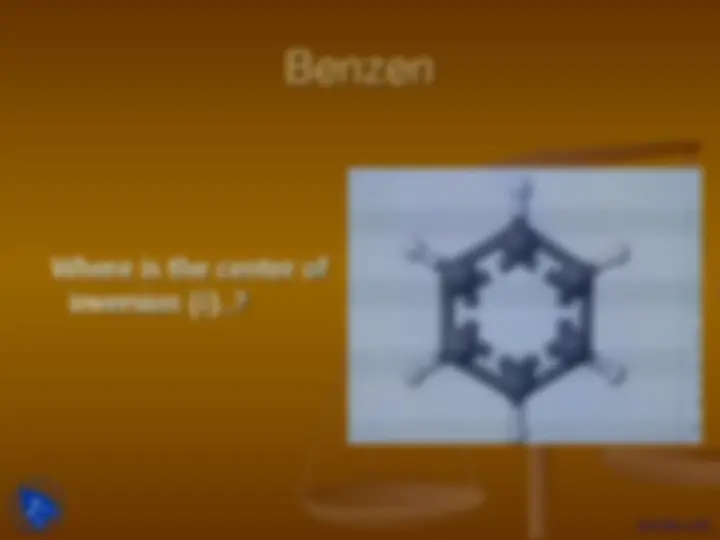
Benzen
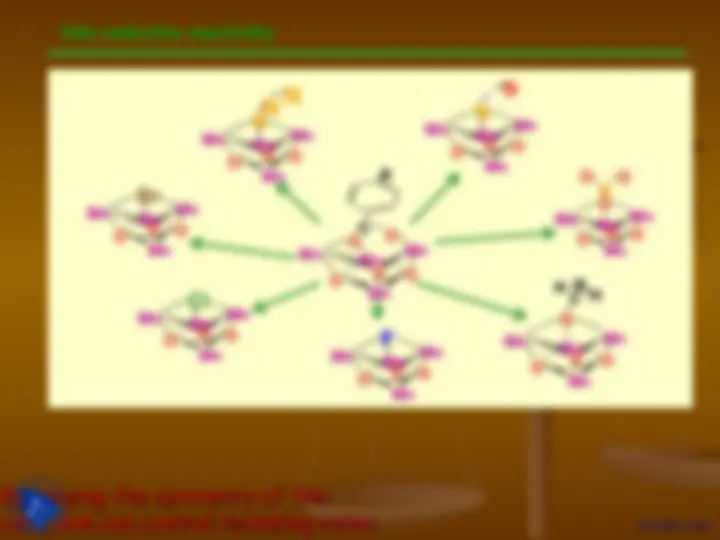
Where is the center of
inversion (i)..?
1 Rotation axes, Cnr
2 Mirror planes , r
3 Inversion centres, I
4 Improper rotations, Snr
5 Translation, t
6 Identity, E
Applications of symmetry
Color
Reflection of light
Birefringence
Pleochroism
Polarization of light
Cleavage
The piezoelectric effect
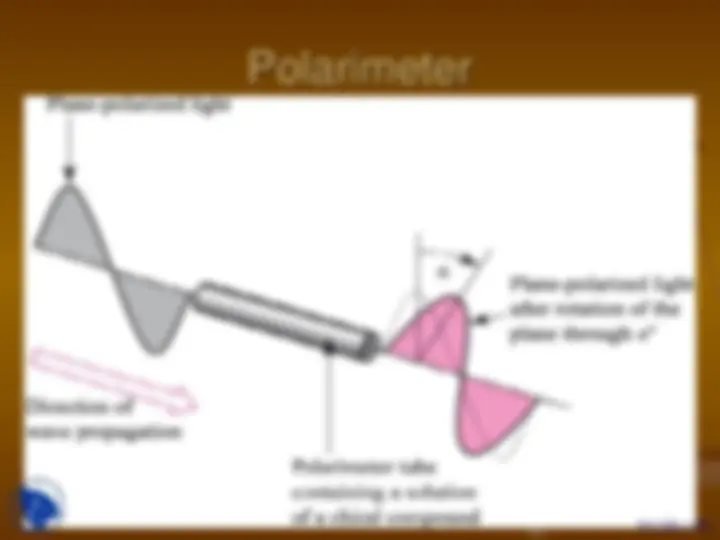
Polarimeter
Difference between
mirror plane and inversion center
The handedness is changed.
One of the more significant applications of
symmetry is the analysis of molecular vibrations. Knowing nothing more than the point group of a molecule, you can predict the number and types of vibrational peaks that will show up in the infrared and Raman spectra. Shown below are two (of three) vibrations of the water molecule. Not surprisingly, they are called the symmetric and antisymmetric stretching modes.
PHYSICAL PROPERTIES OF CRYSTALSColor is caused by the absorption of certain wavelengths of light within the material. Metals, including chrome, iron, cobalt, copper, manganese, nickel, and vanadium, absorb certain wavelengths of light and thus cause coloration. Measurement of color is very subjective, and not usually a good diagnostic for identification because many different materials may have similar colors. Refraction of light through materials change. The velocity of light through a material is inversely related to its index of refraction. For a vacuum, n = 1.0. Most minerals range between 1.4 and 3.2. This property will not be tested. Birefringence is when there are two refractive indices for cross-polarized light entering a material. If one ray enters, two rays emerge (the ordinary and extraordinary). If the material is rotated, the ordinary ray stays still and the extraordinary ray traces a circle about the former. Calcite is a famous source of this effect. Sodium Nitrate exhibits similar abilities. The two exitting rays are always polarized at right angles with respect to each other. Dispersion is the spread in refractive indices of light through a material when its wavelength varies from red to violet. The larger the dispersion, the greater the colors of white light will be separated upon exiting the material. Diamond's dispersion is particularly great, so a play of colors is exhibited. This property will not be tested.
Another color change is due to the presence of artificial or natural light (or often flourescent versus incandescent light). Nickel sulfate shows this kind of color change reminiscent of the near-priceless gemstone alexandrite. Polarization of light does occur in some materials. In nature, it occurs strongly in tourmaline. Placed between a "polarization sandwich," sodium chlorate will permit different colors through as the angle between the polarizers changes. Cleavage occurs when a crystal breaks along certain planes more easily than in any other direction. In nature, a distinction is made between perfect and imperfect cleavage. Perfect cleavage (as in the case of flourite, calcite, and diamond) will always break along a cleavage plane. This makes cutting diamond more chalenging, because the angles needed by the gem cutter might not match cleavage planes. Imperfect cleavage (quartz or beryl) can break in direction. Broken shards of quartz look suspiciously like glass. A shattered octahedron of flourite, however, will produce many smaller pyramids, octahedrons, and tabular octahedrons.
Cleavage of Nickel Sulfate Hexahydrate. The Piezoelectric Effect is when a crystal that is compressed produces a charge-flow and voltage drop across the oposite poles. Rochelle salt and natural quartz exhibit this effect. Working the other way, piezo devices can be expanded by applying a voltage across the oposite poles. Piezo horns and tweeters work this way. Also, piezo devices are used to position scanning tunneling electron microscope probes. Some microphones use piezo devices to convert sound to voltage.
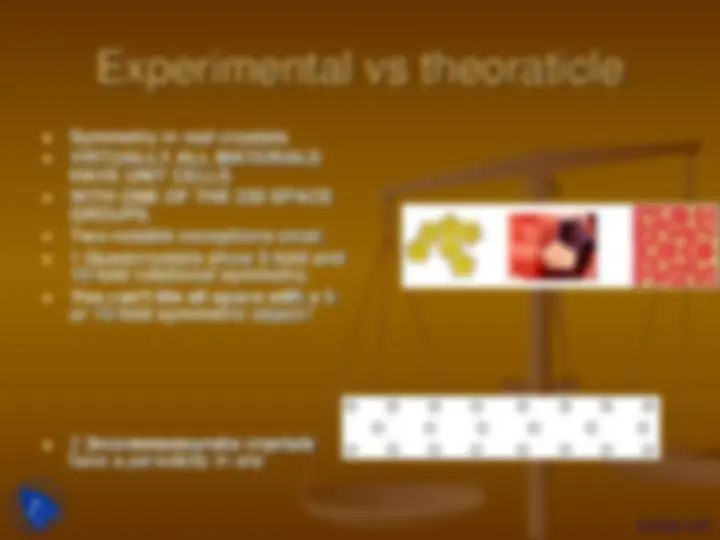
Experimental vs theoraticle
Symmetry in real crystals VIRTUALLY ALL MATERIALS HAVE UNIT CELLS WITH ONE OF THE 230 SPACE GROUPS. Two notable exceptions exist: 1 Quasicrystals show 5-fold and 10-fold rotational symmetry. You can’t tile all space with a 5- or 10-fold symmetric object****!
2 Incommensurate crystals have a periodicity in one
[Ni(hmp)(t-BuEtOH)Cl] 4 Single Crystal
10 -
10 0
10 1
0 5 10 15 20 25 30
(s) at 0.1 M (^) s
^ (s)
1/T (1/K)
= 6.3 x 10 -3^ s * exp[2 K/T]
docsity.com