Download Practical Experiments on Carbohydrate Tests and more Lecture notes Organic Chemistry in PDF only on Docsity!
Qualitative Tests of Carbohydrate-I
BCH 202 [Practical] 1
➢ Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones.
➢ Most , but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of
carbon).
➢ In human body, the D-glucose is used.
➢ Simple sugars ends with – ose.
➢ Biological role:
- Carbohydrates are the key source of energy used by living things.
- Also serve as extracellular structural elements as in cell wall of bacteria and plant.
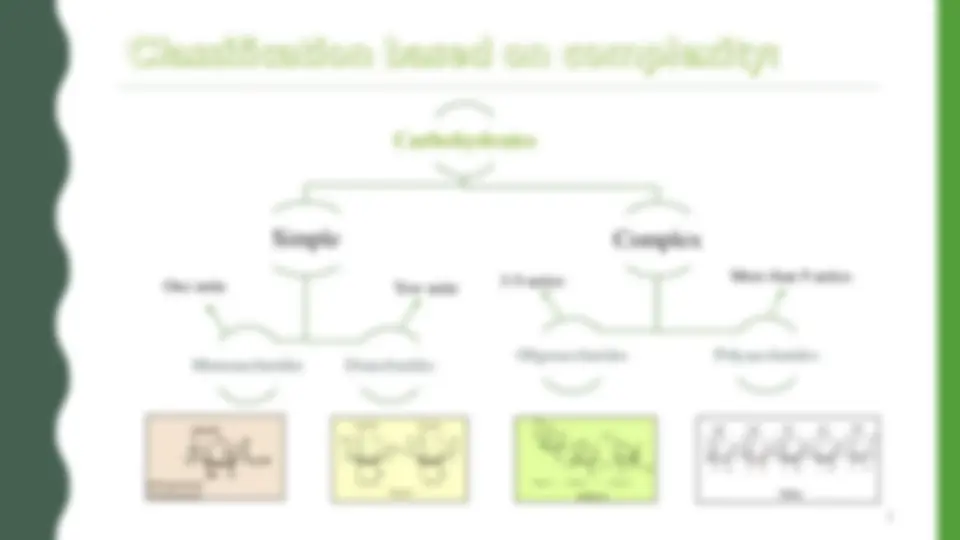
1. Simple sugar (one unit) :
Monosaccharides contain one monosaccharide unit.
2. Complex sugar (more than one) :
1. Disaccharides contain two monosaccharide units.
2. Oligosaccharides contain 3 - 9 monosaccharide units.
3. Polysaccharides can contain more than 9 monosaccharide units.
➢ Complex carbohydrates can be broken down into smaller sugar units through a process
known as hydrolysis.
Carbohydrates Simple Monosaccharides (^) Disaccharides Complex Oligosaccharides Polysaccharides One unite (^) Tow unite 3 -^9 unites^ More than 9 unites
- All monosaccharides are reducing sugars; they all have a free reactive carbonyl group.
- Some disaccharides have exposed carbonyl groups and are also reducing sugars like
lactose. While other disaccharides such as sucrose are non-reducing sugars and will not
react with Benedict's solution.
- Large polymers of glucose, such as starch, are not reducing sugars, since the concentration
of hemiacetal groups is very low.
- Monosaccharide and disaccharid e can be dissolved freely in water because water is a
polar substance.
- Polysaccharide cannot be dissolved easily in water, because, it has high molecular weight ,
which give colloidal solutions in water.
Molicsh test: To identify the carbohydrate from other macromolecules.
Benedict test: For the presence of reducing sugars. Barfoed’s Test: for to distinguish between reducing monosaccharides, reducing disaccharides and non reducing di-polysaccharides (detect reducing monosaccharides). Bial’s Test: To distinguish between pentose monosaccharide and hexose monosaccharide (to detect pentoses). Seliwanoff's Test: To distinguish between aldoses and ketoses (to detect ketoses). 1 2 3 4 5
➢ Objective:
To identify the carbohydrate from other macromolecules lipids and proteins (this test is specific for all
carbohydrates).
➢ Principle:
Two solutions are added : H 2 SO 4 and α-naphthol
1. The test reagent (H 2 SO 4 ) dehydrates pentose to form furfural and dehydrates hexoses to form 5 -
hydroxymethyl furfural.
2. The furfural and 5 - hydroxymethyl furfural further react with α-naphthol present in the test reagent
to produce a purple product.
➢ Method:
1. Two ml of a sample solution is placed in a test tube.
2. 0.5 ml of the Molisch reagent (which α-napthol in
95 % ethanol) is added.
3. The solution is then poured slowly into a tube containing two
ml of concentrated sulfuric acid so that two layers form, producing violet ring appear as
liaison between the surface separations.
➢ Results:
Tube Observation Glucose Lactose Starch Concentrated sulfuric acid is extremely corrosive and can cause serious burns when not handled properly.
➢ Objective:
To distinguish between the reducing and non-reducing sugars (to detect the presence of reducing sugar).
➢ Principle:
- The copper sulfate (CuSO 4 ) present in Benedict's solution reacts with electrons from the aldehyde or
ketone group of the reducing sugar in alkaline medium.
- Reducing sugars are oxidized by the copper ion in solution to form a carboxylic acid and a reddish
precipitate of copper oxide.
- The non-reducing sugars give negative result. reducing sugar carboxylic acid reddish precipitate
16 ➢ Objective: This test is performed to distinguish between reducing monosaccharides, reducing disaccharides and non reducing di- and polysaccharides. ➢ Principle:
- Barfoed’s test used copper (II) ions in a slightly acidic medium.
- Reducing saccharides are oxidized by the copper ion in solution to form a carboxylic acid and a reddish precipitate of copper (I) oxide.
- Different types of reducing sugars react at different rates ➔ Reducing monosaccharides react quickly with Barfoed’s reagent (acidic condition), but reducing disaccharides react very slowly or not at all.
- The non-reducing sugars give negative result. reducing sugar carboxylic acid reddish precipitate
➢ Method:
1. Place one ml of a sample solution in a test tube.
2. Add 3 ml of Barfoed's reagent (a solution of cupric acetate and acetic acid.
3. Heat the solution in a boiling water bath for 6 minutes (after the 3 min check the tubes).
➢ Results:
Tube Observation Glucose Lactose Starch Glucose (+)
➢ Method:
1. Put 2 ml of a sample solution in a test tube.
2. Add 2 ml of Bial's reagent to each tube.
3. Heat the tubes gently in hot water bath.
4. If the color is not obvious, more water can be added to the tube.
➢ Results:
Tube Observation Glucose Ribose
Glucose
Ribose
➢ Objective: To distinguish between aldoses and ketoses (to detect ketoses). ➢ Principle:
- Seliwanoff’s test uses 6 M HCl as dehydrating agent and resoncinol as condensation reagent.
- The test reagent dehydrates ketohexoses to form 5 - hydroxymethylfurfural ➔ 5 - hydroxymethylfurfural further condenses with resorcinol present in the test reagent to produce a cherry red product within two minutes.
- Aldohexoses react to form the same product, but do so more slowly giving yellow to faint pink color.