































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
A comprehensive overview of the fundamental principles and applications of nuclear magnetic resonance (nmr) spectroscopy. It delves into the concept of nuclear magnetic moments, the behavior of nuclei in the presence of an external magnetic field, and the phenomenon of resonance transition between magnetic energy levels. How nmr spectroscopy operates by applying a magnetic field to nuclei and measuring the energy required to bring them into resonance. It also discusses the factors that influence the chemical shift, such as shielding and deshielding, anisotropic effects, and the splitting of signals due to spin-spin coupling. The practical applications of nmr spectroscopy in various fields, including organic chemistry, biochemistry, and medical imaging. Overall, this document serves as a valuable resource for students and researchers interested in understanding the principles and applications of this powerful analytical technique.
Typology: Summaries
1 / 47

This page cannot be seen from the preview
Don't miss anything!








































Applied Chemistry-B CYCI-102, CSE
NIT Jalandhar
2021
Dr Singh
Radio waves have the lowest energy of the various kinds of electromagnetic radiation. We use them for radio and television
communication, digital imaging, remote control devices, and wireless linkages for computers. Radio waves are also used in
NMR spectroscopy and in magnetic resonance imaging (MRI).
Image and notes from Organic chemistry, P Y Bruice
2
Consider simple example of Hydrogen atom , as it consist of one electron and one
proton
a. So one electron circulating around hydrogen (Figure 1)
b. And the hydrogen has a spin as shown in the figure (Figure 2)
c. We can consider hydrogen as a bar magnet (Figure 3), so hydrogen behaves a
magnet
Many atomic nuclei have a property called spin: the nuclei behave as if they were spinning.
In fact, any atomic nucleus that possesses either odd mass, odd atomic number, or both has a quantized spin
angular momentum and a magnetic moment
(Figure 1)
(Figure 2) (Figure 3)
All molecules with non-zero spin have a magnetic moment
= l
Here is magnetic moment, gyromagnetic ratio and l is the angular momentum
Because of magnetic moment of the nucleus, forces nucleus to behave as a tiny bar magnet
Nucleus Spin gyromagnetic ratio Natural Abundance
1
13
Representing the magnet
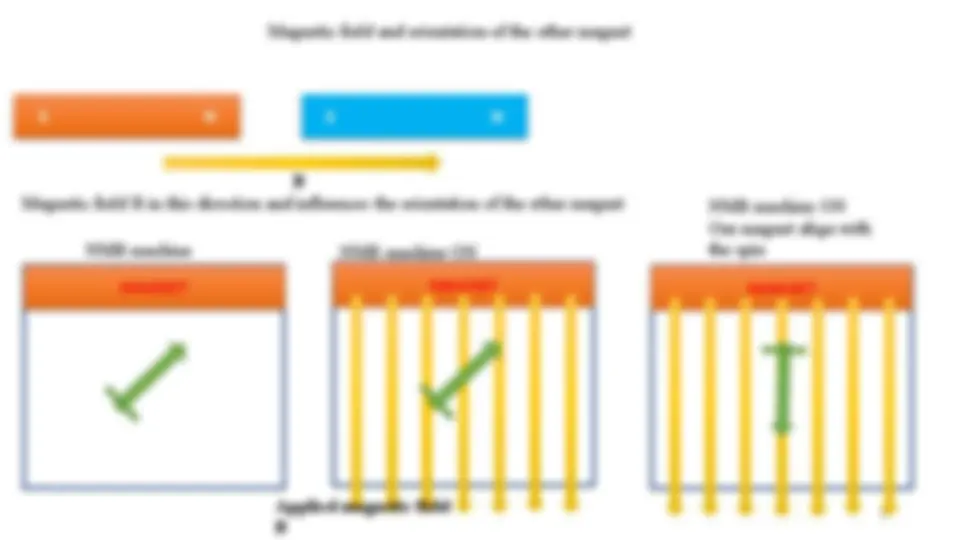
What happens when we bring two magnets together
Repulsion and attraction
Magnetic field and orientation of the other magnet
Magnetic field B in this direction and influences the orientation of the other magnet
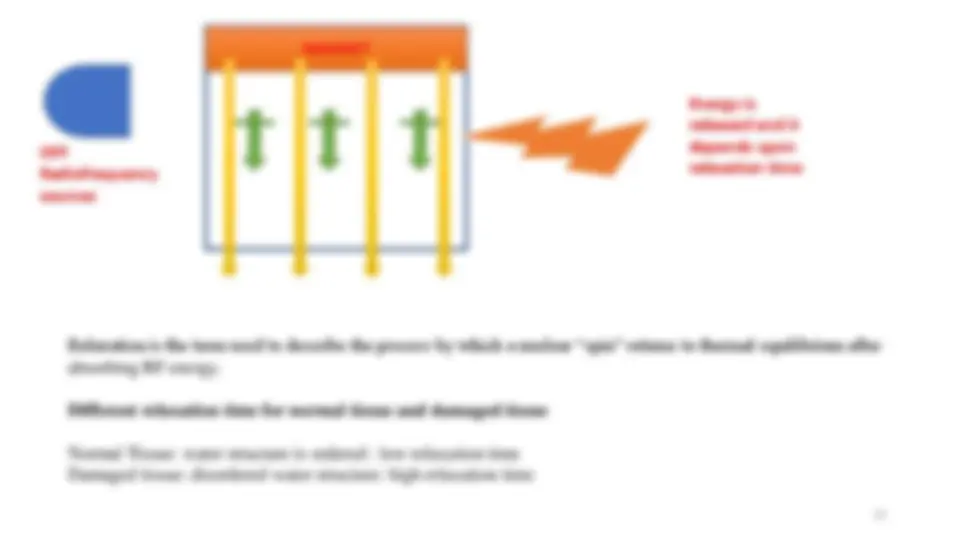
NMR machine ON
Our magnet align with
the spin
NMR machine
Applied magnetic field
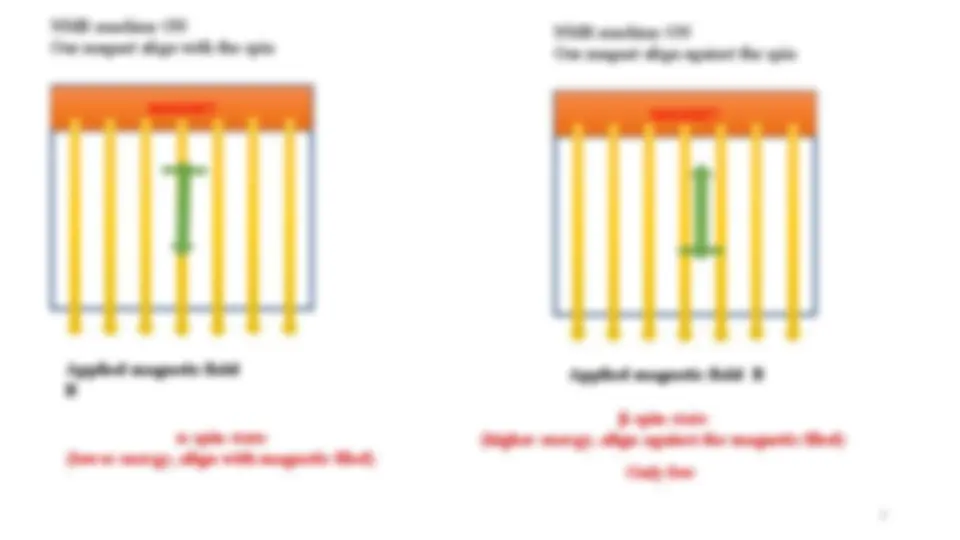
NMR machine ON
8
The number of allowed spin state of a spinning nucleus can be determined from spin quantum number I
Where allowed spin states are 2I+1 with integral difference ranging from + I to – I
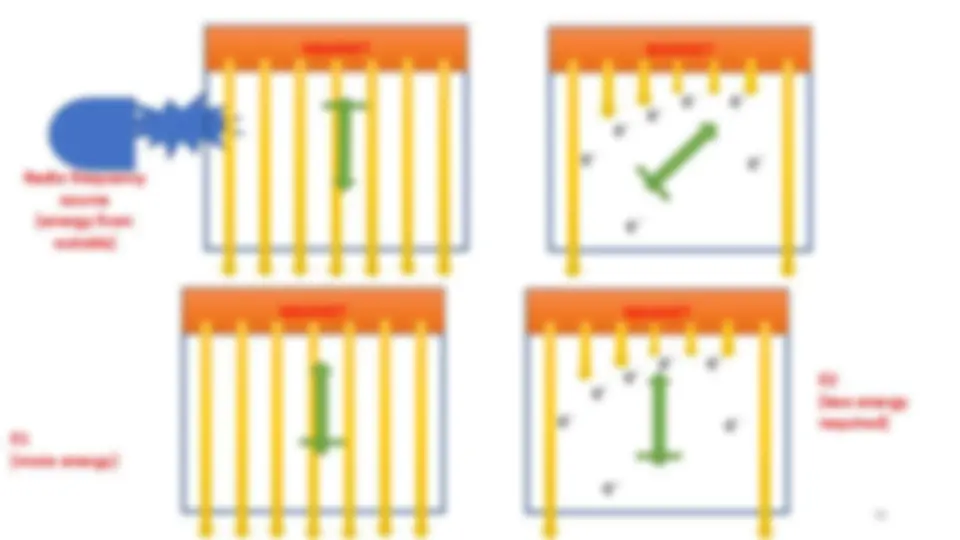
For proton I = ½ so allowed transitions will be ====== 2I+1 so 2 *1/2 + 1 = 2, i.e. +1/2 to - 1/
In the absence of applied magnetic field all spin states of a given nucleus are of equivalent energy (degenerate)
Hydrogen atom (proton) in different environment
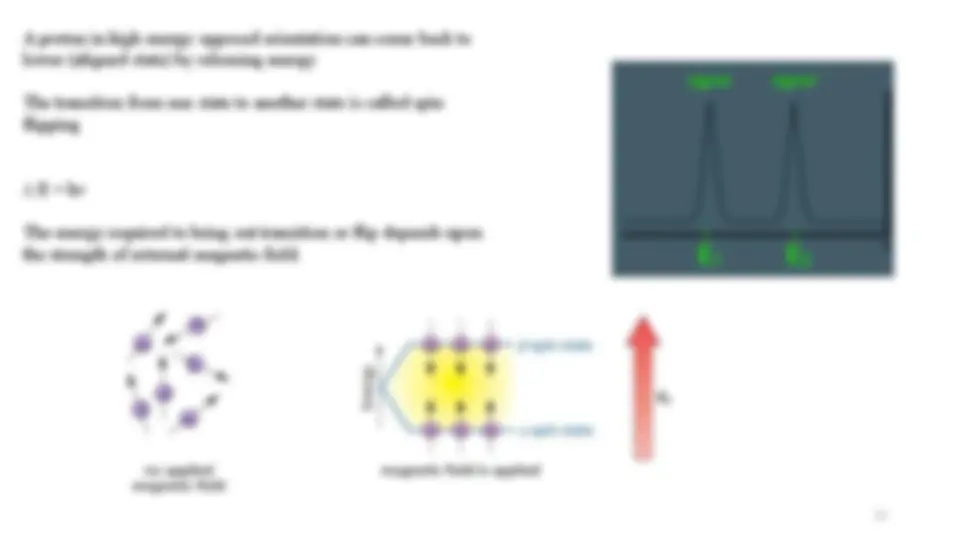
A proton in high energy opposed orientation can come back to
lower (aligned state) by releasing energy
The transition from one state to another state is called spin
flipping
E = hv
The energy required to bring out transition or flip depends upon
the strength of external magnetic field.
14
Image and notes from Organic chemistry, P Y Bruice
depends upon the type of nucleus
involved (which depends upon the
gyromagnetic ration), and chemical
environment
Proton is considered as a child’s spinning top
Proton: like a spinning top
The phenomenon of precession is similar to that of a spinning top. Owing to the influence of the earth’s gravitational
field, the top begins to “wobble,” or precess, about its axis. A spinning nucleus behaves in a similar fashion under the
influence of an applied magnetic field (as shown in next slide).
17
A spinning nucleus “wobble,” under the influence of an applied
magnetic field
The precession of a spinning nucleus resulting
from the influence of an applied magnetic field
A top precessing in the earth’s gravitational field
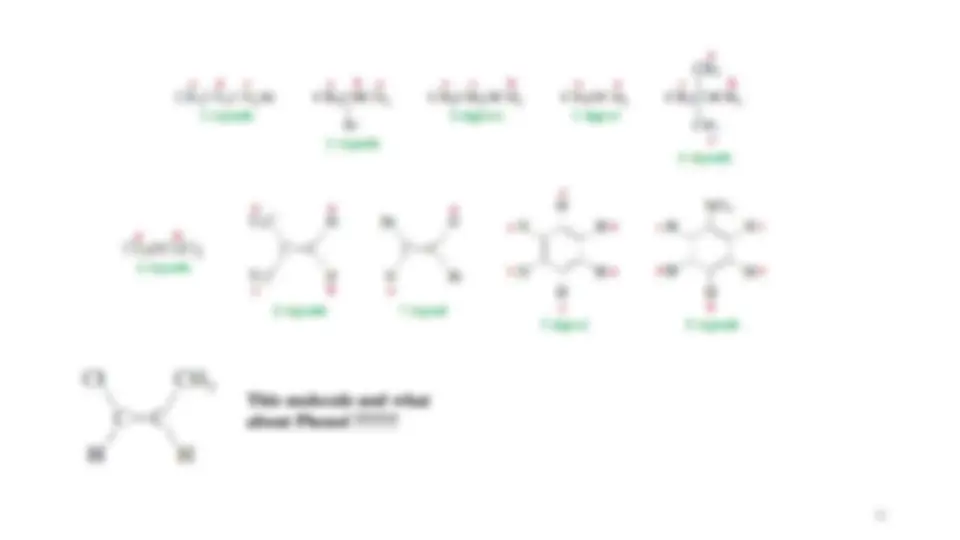
ClCH
2
CH
3
In a magnetic field, the electrons circulate about the nuclei and induce a local magnetic field that acts in opposition to the
applied magnetic field and, therefore, subtracts from it. As a result, the effective magnetic field —the amount
of magnetic field that the nuclei actually “sense” through the surrounding electrons—is somewhat smaller than the applied
magnetic field:
Diamagnetic shielding
The protons in electron-rich environments sense a smaller
effective magnetic field.
Therefore, they require a lower frequency to come into
resonance—that is, flip their spin—because E is smaller (
Figure). Protons in electron-poor environments sense
a larger effective magnetic field and so require a higher
frequency to come into resonance
because E is larger.
ClCH
2
CH
3