
















































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
The main points are: Nonlinear Dielectrics, Coupling of Strain, Piezoelectric Ceramics, Temperature Dependence of Polarization, Absence of Electric Field, Ferroelectric Ceramics, Classification Based on Crystal Classes, Pyroelectric Ceramics
Typology: Study notes
1 / 55

This page cannot be seen from the preview
Don't miss anything!
















































So far, we have discussed linear dielectrics whose dielectric constant increases linearly with the applied field accompanied by an increase in the polarization depending upon the presence of polarization mechanisms in the materials.
In addition, there are a few special classes of dielectric materials which show large dielectric constants, non-zero polarization in the absence of electric field and nonlinearity in the dielectric constant. These also show extraordinary special effects such as
Coupling of strain and electric field (piezoelectric ceramics),
Temperature dependence of the polarization (pyroelectric ceramics) and
Presence of large polarization in absence of electric field i.e. spontaneous polarization (ferroelectric ceramics).
Most of these materials happen to be oxides and as you can very well understand now, these properties will be greatly affected by the defect chemistry and process variables.
The presence of these features makes these materials extremely useful for a variety of applications such as sensors, actuators, transducers, temperature detectors, imaging, permanent data storage etc. In this module, we will discuss origin of these properties with a crystallographic and thermodynamic framework and associated mathematical representations along with a few examples of materials and devices.
Principles of Electronic Ceramics, by L. L. Hench and J. K. West, Wiley
Principles and applications of ferroelectrics and related materials, M. E. Lines and A. M. Glass, Oxford University Press
Electroceramics: Materials, Properties, Applications, by A. J. Moulson and J. M. Herbert, Wiley
Out of a total of 32 crystal point groups (see the course related to structure of materials), 21 are non-centrosymmetric i.e. crystals not having a center of symmetry.
The term centrosymmetric refers to a space group which contains an inversion center as one of its symmetry elements i.e. for every point (x, y, z) in the unit cell, there is an indistinguishable point (- x, -y, -z).
Cubic m3 m3m none 432 m 23
Tetragonal 4 or m
4 or mmm 4 4mm 2 m 22
Orthorhombic mmm mm2 222
Hexagonal 6 or m
6 or mmm 6 6mm 2m 622
Trigonal m 3 3m 32
Monoclinic 2 or m 2 m none
Triclinic 1 none
Total Number 11 groups 10 groups 11 groups
Out of these 21 point groups, except group 432, crystals containing all other point groups exhibit piezoelectric effect i.e. upon application of an electric field, they exhibit strain or upon application of an external stress, charges develop on the faces of crystal resulting in an induced electric field.
Out of these 20 non-centrosymmetric point groups, 10 belong to polar crystals i.e. crystals which possess a unique polar axis, an axis showing different properties at the two ends.
These crystals can be spontaneously polarized and polarization can be compensated through external or internal conductivity or twinning or domain formation.
Spontaneous polarization depends upon the temperature. Consequently, if a change in temperature is imposed, an electric charge is developed on the faces of the crystal perpendicular to the polar axis. This is called pyroelectric effect. All 10 classes of polar crystals are pyroelectric.
In some of these polar non-centrosymmetric crystals, the polarization along the polar axis can be reversed by reversing the polarity of electric field. Such crystals are called ferroelectric i.e. these are spontaneously polarized materials with reversible polarization.
So by default, all ferroelectric materials are simultaneously pyroelectric and piezoelectric. Similarly, all pyroelectric materials are by default piezoelectric but not all of them are ferroelectric.
Ferroelectric effect was first observed in Rochelle salt KNaC 4 H 4 O 6 .4H 2 O by Czech scientist Roger Valasek in 1921. Afterwards, for years the discovery did not raise much excitement possibly due to the world war. However, after a few decades, renewed technological interest led to much more extensive studies and a better understanding.
In the subsequent years, many materials were discovered and were of technological interest as they were employed into a variety of applications. Among various categories of ferroelectric materials following stand out either due to interest in the structure or in the properties:
Tri-glycine sulfate and isomorphous materials
Pottasium dihyrogen phosphates and isomorphous materials
Barium titanate and other perovskite structured compounds such as KNbO 3 , PbTiO 3 etc
Complex oxides such as Aurrivillious compounds
Rochelle Salt and similar compounds
Ferroelectric Sulfates
Among the above, perovskite related compounds have been studied most, primarily due to their exciting properties and reasonably high transition temperatures, making them attractive for various applications.
In the following section, we will look some of the structural and thermodynamic features of these materials, with main emphasis on perovskite compounds for the sake of illustration.
file:///C|/Documents%20and%20Settings/iitkrana1/Desktop/new_electroceramics_14may,2012/lecture25(28)/25_4.html[5/25/2012 12:57:57 PM]
These materials consist of net permanent dipole moment i.e. finite vector sum of dipole moment even in the absence of electric field. This requires the material to be non-centrosymmetric whereas dipole moment would be forced to be zero in a centrosymmteric material due to symmetry considerations.
On top of this, there must be a spontaneous polarization as well which means the centers of positive and negative charges in a crystal would never be the same.
The following is the figure showing the crystal structure of a perovskite structured material such as BaTiO 3.^ Here^ in^ cubic^ structure^ (A),^ the^ dipole^ is^ zero^ while^ in^ tetragonal form (B), the dipole moment is finite. This displaced position of the central atom is the energetically stable position i.e. the free energy is minimum.
When this kind of ferroelectric material is switched i.e. subjected to a bipolar electric field, it exhibits a polarization vs electric field plot as shown below. You can see that there is finite polarization in the absence of electric field i.e. two equal and opposite values, +P (^) r or -P (^) r also called remnant polarization. These two values can be connected with the position of a central atom in the octahedra in the figure above. So, when you apply field in the direction, the central atom moves up with respect to oxygen octahedral and when you change the polarity, it comes down in the opposite direction, the extent of displacement being similar.
The plot shows a hysteresis which is important for applications such as memory devices. We will explain the details of this phenomenon in the subsequent sections.
The movement of central atom in the above structure can explained in terms of a potential energy diagram.
This situation can be explained in terms of a potential energy between two adjacent low energy sites. There are two equilibrium positions in which a B ion can stay, but to change from one state to another, energy must be provided to overcome an energy barrier ΔE. These energy wells further tilt to the left or right, depending upon the polarity of the electric field i.e. in the non-zero field state making on configuration more stable than another. However, a coercive field, which is the field required to bring the polarization back to zero, is needed when you switch to other direction of the field.
Ferroelectric materials follow Curie-Weiss law which is expressed as
Here, N is the number of dipoles per unit volume of the material, a is dipolar polarizability, and T (^) c is defined as the Ferroelectric transition temperature or the Curie temperature.
Ferroelectric behaviour is observed below the Curie Temperature above which the ferroelectric phase converts into a paraelectric phase which always has higher symmetry than the ferroelectric phase (for example transformation of low symmetry tetragonal BaTiO 3 to higher symmetry cubic BaTiO 3 at about 120^ o^ C while heating) and ferroelectricity disappears. A paraelectric state is essentially a centrosymmetric higher symmetry state where dipoles are randomly oriented in a crystal giving rise to zero polarization.
Below this T (^) c spontaneous polarization is prevalent and dipoles tend to align. So, since C = a (^) d kT,
we get
Thus, by now modifying equation (5.4), Curie-Weiss law can be expressed as
Thermodynamic theory to understand the ferroelectric phase transitions was developed after contributions from Lev Landau and V.L. Ginzburg, both Soviet physicists and A.F. Devonshire, British Physicist. The approach is based around calculating the free energy of system and working around other thermodynamic parameters to predict the nature of phase transition. Here, before we go into details of this theory, we will look at the some of the basic definitions.
These non-linear dielectrics exhibit various kinds of couplings between physical properties and can be expressed mathematically. For instance, below are the expressions for ferroelectric, piezoelectric
Electric charge in a polar material can be induced by application of an external electric field and can be expressed as
where χij is the susceptibility in F/m (actually dimensionless but here e (^) o i.e. permittivity of free space is also included which has dimension of F/m) and is second rank tensor. Note that the equation is valid only for the linear region of the hysteresis curve.
Similarly, the charge induced by application of external stress i.e. piezoelectric effect, can be expressed by
where is the piezoelectric coefficient and is third rank tensor with units C/N, is the stress applied.
Converse piezoelectric effect is expressed as (5.10)
Where d (^) ijkt^ is in m/V.
Induced charge by temperature change i.e. pyroelectric effect is expressed by
where P (^) i is the vector of pyroelectric coefficient in cm -2^ K -^.
Displacement is expressed as
which is the same expression that we encountered for linear dielectrics.
According to Landau-Devonshire theory, near the Curie point (T~T 0 ) we assume
a = a 0 (T - T 0 ) (^) (5.19)
As a result, the free energy expansion (5.13), only ‘a’ is dependent on temperature while other constants are temperature independent. Incorporating (5.18) into (5.13) yields
In this expression, for all known ferroelectrics, both ao and c are positive while depending upon the sign of b, the phase transition nature changes.
Figure 5.6 (a) show the free energy vs polarization plot when T >> T 0 i.e. when the crystal is in
paraelectric state. On the other hand if, a < 0 when T << T 0 and b and c are positive, there will be a
nonzero root of P in addition P = 0, as shown below in figure 5.6 (b), representing the ferroelectric state with non-zero polarization at zero field.
When b is positive, the ferroelectric transition occurs at a temperature T = T 0 and is called as second order transition (do not get confused between T 0 and T (^) c as the distiction will become clear in the next section when we learn first order phase transition). Under such a situation, free energy as function of polarization evolves continuously when temperature is changes i.e. from a curve with single minima at P = 0 when T < T 0 to a plot with two minima at P = +P 0 and –P 0 when T > T 0. Two curves become closely related if ‘a’ changes continuously with temperature and changes sign at T (^) c and can be shown together as in figure 5.7 (a) below.
The spontaneous polarization, P 0 , can be estimated by substituting E = 0 in equation (5.20) and retaining only two lowest order terms since all the coefficients i.e. a 0 , b and c are positive. The polarization can be expressed as
showing that the polarization decreased to zero at T = T 0 as shown in figure 5.8.
Dielectric susceptibility at T < T 0 can be estimated as
Showing that susceptibility will have a divergence at T = T 0 or its reciprocal (i.e. dielectric stiffness) will vanish at T = T 0 as shown in figure 5.8. In real materials, susceptibility reaches very large values near T 0.
Another situation to consider is that when a < 0, b < 0 but c > 0. What this means is that free energy vs polarization plot has three equal minima, one for P = 0 and the other two for P ≠ 0 at the same temperature i.e. at the same value of ‘a’ at a temperature T = T 0 , Curie temperature, which is now more than T 0. This gives rise to the following free energy vs polarization plot.
The most important feature of this phase transition is that polarization i.e. the order parameter drops from P ≠ 0 to zero discontinuously at T = T (^) c and is called as first order phase transition_._^ This is also very clearly demonstrated by a discontinuity in the reciprocal of dielectric susceptibility as seen below. For example, solid-liquid phase transition is a first order phase transition while among various ferroelectrics, barium titanate is a fine example of first order transition among ferroelectrics.
In order to compute the non-zero polarization (P 0 ) and susceptibility at the transition, the value of free energy (5.13) for P = 0 and P = P 0 must be equal at T = T (^) c i.e.
On the other hand, field E must also be zero for the polarization to be spontaneous i.e.
The polarization and susceptibility at T (^) c are obtained by solving two equations and are given as
and
(5.28)
The fact that there are three minima at T=T (^) c is reflected in whether the T (^) c is approached while
heating or cooling. More specifically, the material will be in other of the two non-zero polarization states if is heated from an initial temperature that is lower than T (^) c whereas, if it is cooled from a
temperature higher than T (^) c , the sample will be in paraelectric state. This results in thermal
hysteresis when these materials are thermally cycled across T (^) c.
If you are interested in further reading about the phase transitions in ferroelectrics, refer to the following texts:
Principles and Applications of Ferroelectrics and Related Materials, M. Lines and A. Glass, Clarendon Press, Oxford
Solid State Physics, A.J. Dekker, Macmillan Publishing
Ferroelectric Crystals, F. Jona and G. Shirane, Dover Publishing
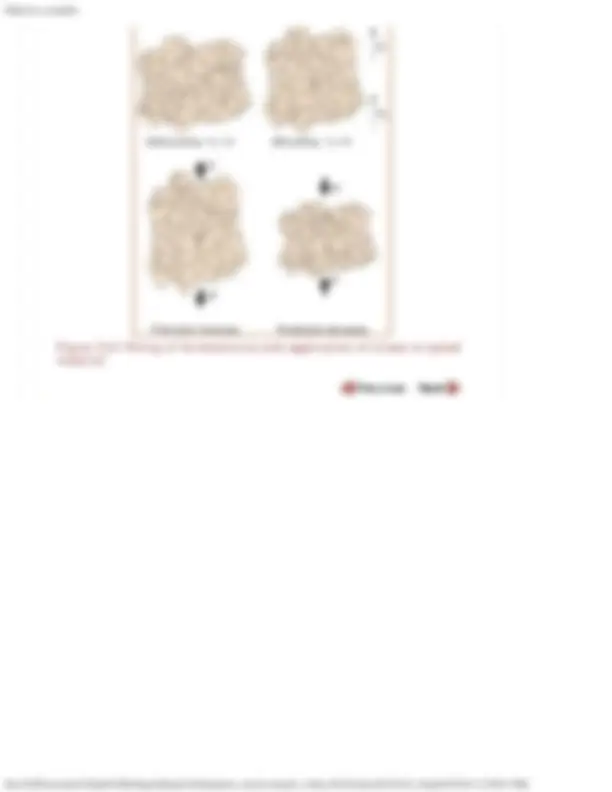
Domains can also be seen by microscopy. The following is an image of domains in BaTiO 3 as seen
by transmission electron microscopy.
Ferroelectric domains form as a result of instabilities due to alignment of dipoles in a crystal. The free energy change involved in the formation of a domain is given as
(5.29)
where U (^) c : effect of applied field on the domain energy U (^) p and U (^) x : bulk electrical and elastic energies U (^) d : depolarization energy and U (^) w : domain energy U (^) d is the energy related to the internal field set up in the crystal by the polarization and not compensated. The internal field opposes the applied field E and hence is called as depolarizing fieldThis is dependent on the domain size (d) and is expressed as
Here e *^ is a constant determined by the dielectric constant of the material, t is crystal thickness, P (^0) is the polarization at the center of the domain.
U (^) w is the domain energy and can be expressed in terms of surface energy of the wall (γ), domain width (d) and crystal volume (V) and is given as
To get a stable domain structure, this ΔG has to be minimized. In (5.29), U (^) p and U (^) x are the same in each domain, irrespective of domain thickness and so are independent of domain size and hence are treated as constant. Therefore one only needs to consider U (^) w and U (^) d when ΔG is minimized w.r.t. domain wall thickness d i.e. dΔG/d d = 0 resulting in
Here, one can see that domain size is dependent on surface energy, crystal thickness and polarization, showing a competition between the surface energy of the wall and polarization (governing the depolarizing field). This shows that larger the surface energy is, larger the domain size is which makes sense because of large energy requirement, the interface area needs to be smaller. Secondly, the larger the polarization is, the larger the depolarizing field will be and large would be the driving force for the domains to form and hence smaller domains will be preferred.