































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Material Science for Engineers which includes Structure of Wood, Moisture Content, Density of Wood, Mechanical Properties of Wood, Expansion and Contraction of Wood, Concrete Materials, Properties of Concrete etc. Key important points are: Nonferrous Alloys, Aluminum Alloys, Magnesium and Beryllium Alloys, Copper Alloys, Nickel and Cobalt Alloys, Titanium Alloys, Refractory and Precious Metals, Temper Designation, Hall-Heroult Process
Typology: Slides
1 / 38

This page cannot be seen from the preview
Don't miss anything!































Chapter 13 – Nonferrous Alloys
Section 13.
Aluminum Alloys
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning
is a trademark used herein under license.™
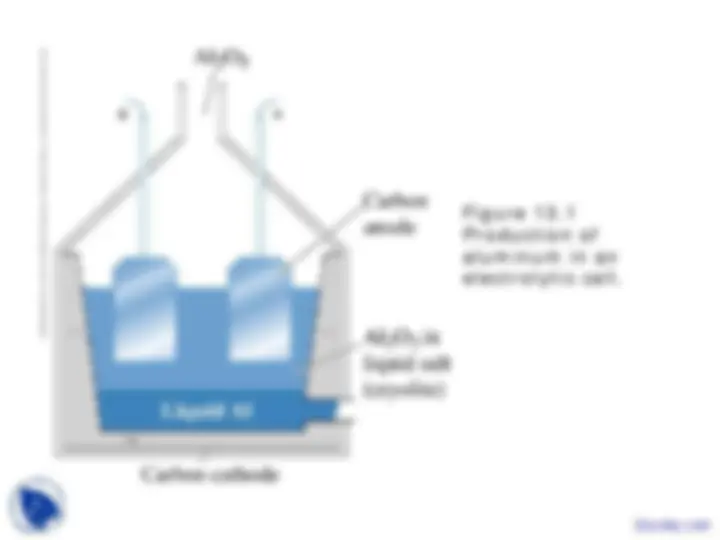
Figure 13. Production of aluminum in an electrolytic cell.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
Figure 13.3 Portion of the aluminum-magnesium phase diagram.
Figure 13.4 (a) Sand-cast 443 aluminum alloy containing coarse silicon and inclusions. (b) Permanent-mold 443 alloy containing fine dendrite cells and fine silicon due to faster cooling. (c) Die-cast 443 alloy with a still finer microstructure ( × 350). ( From ASM Handbook, Vol. 7, (1972), ASM International, Materials Park, OH 44073 .)
Example 13.1 SOLUTION
a. Load = F = σy × A = 70.000 ( π /4) (0.5 in.) 2 = 13,744 lb
b. The yield strength of the aluminum alloy is 36,000 psi. Thus: A = ( π/ 4)d^2 = F/ σy = 13,744/36,000 = 0.38 in.^2 d = 0.697 in. Density of steel = ρ = 7.87 g/cm^3 = 0.284 lb/in.^3 Density of aluminum = ρ = 2.70 g/cm^3 = 0.097 lb/in 3
c. Weight of steel = Al ρ = ( π /4)(0.5in) 2 (12)(0284) = 0.669 lb/ft Weight of aluminum = Al ρ = ( π /4)(0.697) 2 (2) (12) (0.097) = 0.444 lb/ft Although the yield strength of the aluminum is lower than that of the steel and the cable must be larger in diameter, the aluminum cable weighs only about half as much as the steel cable. When comparing materials, a proper factor-of- safety should also be included during design.
Design a method for recycling aluminum alloys used for beverage cans.
Example 13.2 SOLUTION
One approach to recycling the cans is to separate the two alloys from the cans. The cans are shredded, then heated to remove the lacquer that helps protect the cans during use. We could then further shred the material at a temperature where the 5182 alloy begins to melt. The small pieces of 5182 can therefore be separated by passing the material through a screen. The two separated alloys can then be melted, cast, and rolled into new can stock.
An alternative method would be to simply remelt the cans. Once the cans have been remelted, we could bubble chlorine gas through the liquid alloy. The chlorine reacts selectively with the magnesium, removing it as a chloride. The remaining liquid can then be adjusted to the proper composition and be recycled as 3004 alloy.
Example 13. Design of an Aluminum Recycling Process
Design a casting process to produce automotive wheels having reduced weight and consistent and uniform properties.
Example 13.4 SOLUTION
Thixocasting process in which the material is stirred during solidification, producing a partly liquid, partly solid structure that behaves as a solid when no external force is applied, yet flows as a liquid under pressure. We would select an alloy with a wide- freezing range so that a significant portion of the solidification process occurs by the growth of dendrites. A hypoeutectic aluminum-silicon alloy might be appropriate. In the thixocasting process, the dendrites are broken up by stirring during solidification. The billet is later reheated to cause melting of just the eutectic portion of the alloy, and it is then forced into the mold in its semi-solid condition at a temperature below the liquidus temperature.
Example 13. Design of a Casting Process for Wheels
Magnesium alloys are used in aerospace applications, high-speed machinery, and transportation and materials handling equipment.
Instrument grade beryllium is used in inertial guidance systems where the elastic deformation must be minimal; structural grades are used in aerospace applications; and nuclear applications take advantage of the transparency of beryllium to electromagnetic radiation. Beryllium is expensive, brittle, reactive, and toxic.
Section 13.
Magnesium and Beryllium Alloys
Blister copper - An impure form of copper obtained during the copper refining process.
Applications for copper-based alloys include electrical components (such as wire), pumps, valves, and plumbing parts, where these properties are used to advantage.
Brass - A group of copper-based alloys, normally containing zinc as the major alloying element.
Bronze - Generally, copper alloys containing tin, can contain other elements.
Section 13.
Copper Alloys
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning
is a trademark used herein under license.™
Figure 13. Binary phase diagrams for the (a) copper-zinc, (b) copper-tin, (c) copper- aluminum, and (d) copper- beryllium systems.
Design the heat treatment required to produce a high-strength aluminum-bronze gear containing 10% Al.
Example 13. Design of a Heat Treatment for a Cu-Al Alloy Gear
Figure 13.6 Binary phase diagrams for the (c) copper-aluminum
Example 13.6 SOLUTION