








Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Science and Engineering of Materials which includes Point Defects, Types of Defects, Equilibrium Number, Thermal Vibrations, Boltzmann Constant, Regular Lattice Sites, Substitutional Solid Solutions, Composition Conversions etc. Key important points are: Materials Science, Classification of Materials, Historical Perspective, Electrical and Magnetic Properties, Chemical Stability, Types of Materials, Material Selection, Future of Materials Science`
Typology: Slides
1 / 13

This page cannot be seen from the preview
Don't miss anything!








Stone → Bronze → Iron → Advanced materials
Processing → Structure → Properties → Performance
Metals, Ceramics, Polymers, Semiconductors
Electronic materials, superconductors, etc.
Biodegradable materials, Nanomaterials, “Smart” materials
Material science is the investigation of the relationship among processing, structure, properties, and performance of materials.
Electronic structure of individual atoms that defines interaction among atoms (interatomic bonding).
Arrangement of atoms in materials (for the same atoms can have different properties, e.g. two forms of carbon: graphite and diamond)
Arrangement of small grains of material that can be identified by microscopy.
Structural elements that may be viewed with the naked eye.
Monarch butterfly ~ 0.1 m
Properties are the way the material responds to the environment and external forces.
Mechanical properties – response to mechanical forces, strength, etc.
Electrical and magnetic properties - response electrical and magnetic fields, conductivity, etc.
Thermal properties are related to transmission of heat and heat capacity.
Optical properties include to absorption, transmission and scattering of light.
Chemical stability in contact with the environment - corrosion resistance.
Let us classify materials according to the way the atoms are bound together (Chapter 2).
Metals: valence electrons are detached from atoms, and spread in an 'electron sea' that "glues" the ions together. Strong, ductile, conduct electricity and heat well, are shiny if polished.
Semiconductors: the bonding is covalent (electrons are shared between atoms). Their electrical properties depend strongly on minute proportions of contaminants. Examples: Si, Ge, GaAs.
Ceramics: atoms behave like either positive or negative ions, and are bound by Coulomb forces. They are usually combinations of metals or semiconductors with oxygen, nitrogen or carbon (oxides, nitrides, and carbides). Hard, brittle, insulators. Examples: glass, porcelain.
Polymers: are bound by covalent forces and also by weak van der Waals forces, and usually based on C and H. They decompose at moderate temperatures (100 – 400 C), and are lightweight. Examples: plastics rubber.
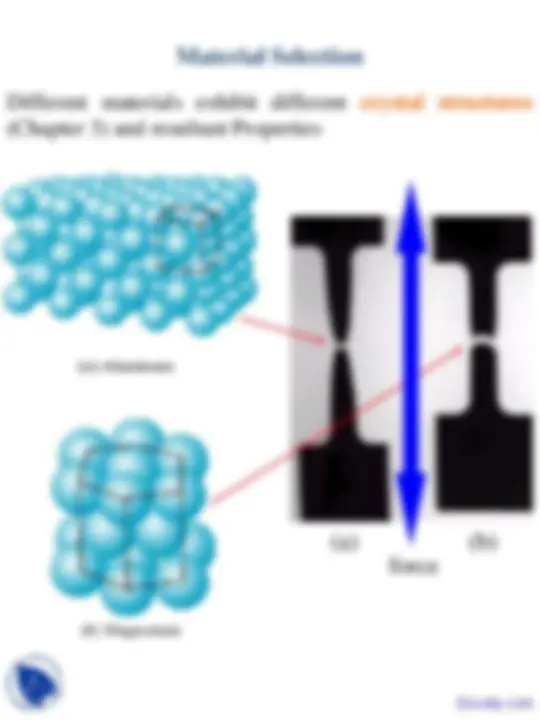
Different materials exhibit different microstructures (Chapter 4) and resultant Properties
Superplastic deformation involves low-stress sliding along grain boundaries, a complex process of which material scientists have limited knowledge and that is a subject of current investigations.
How do you decide on a specific material for your application?
Design of materials having specific desired characteristics directly from our knowledge of atomic structure.