




































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
basic engginerring lasers
Typology: Thesis
1 / 49

This page cannot be seen from the preview
Don't miss anything!










































2 1
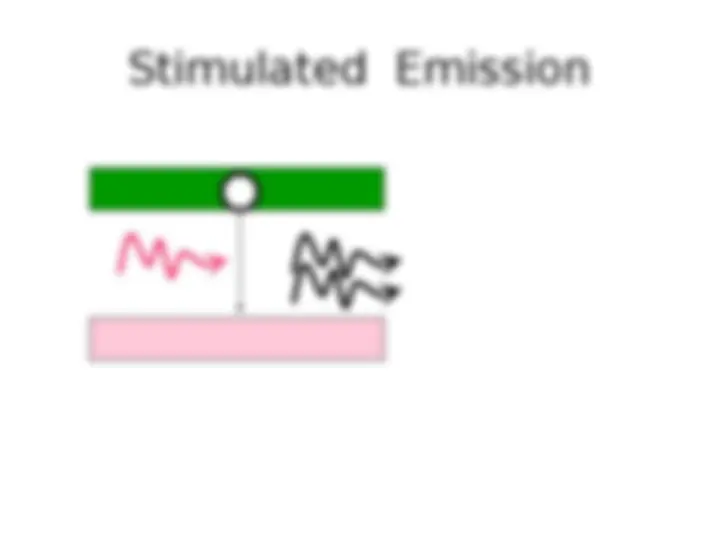
atoms in an upper energy level can be triggered or stimulated in phase by an incoming photon of a specific energy. 2 1
E 1 E 2 h (a) Absorption h (b) Spontaneous emission h (c) Stimulated emission In h Out h E 2 E 2 E 1 E 1 Absorption, spontaneous (random photon) emission and stimulated emission. © 1999 S.O. Kasap, Optoelectronics (Prentice Hall)
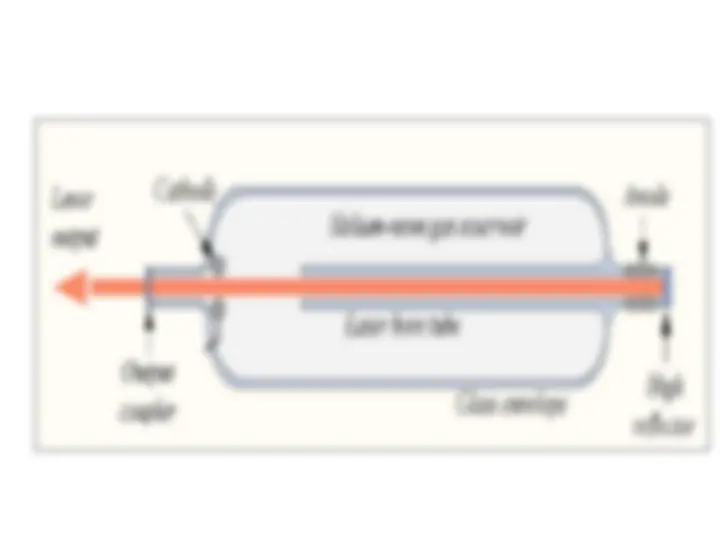
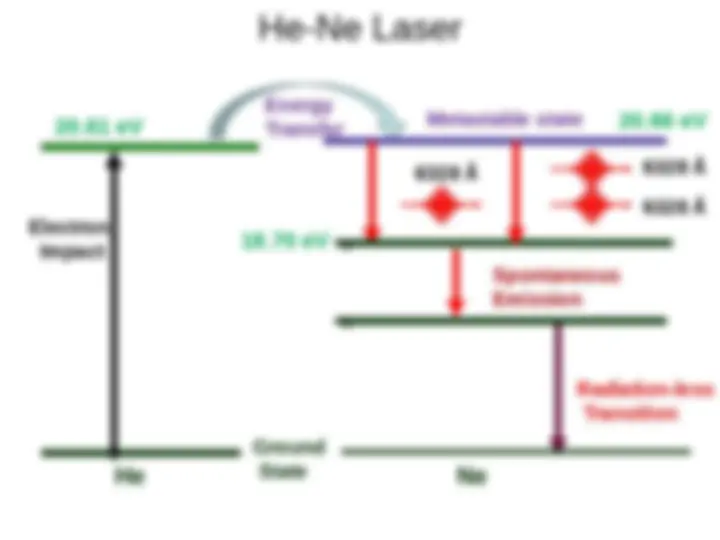
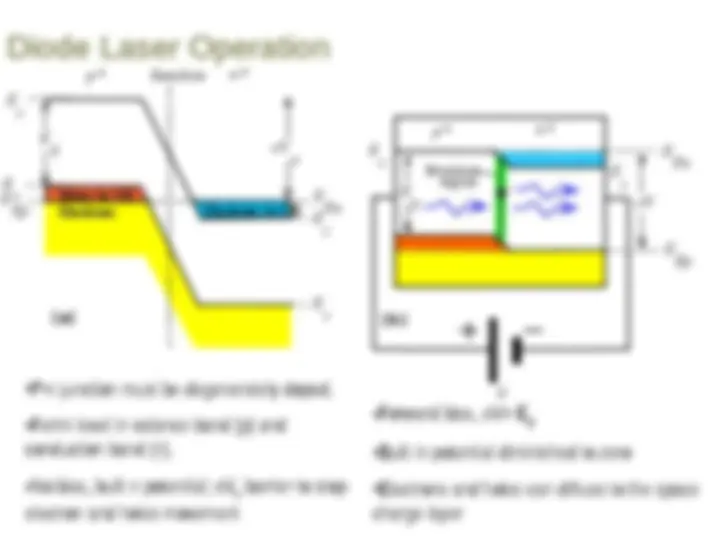
Stimulated emission leads to a chain reaction and laser emission Excited medium If a medium has many excited molecules or atoms, one photon can become many. This is the essence of the laser.
Let N 1 and N 2 be the number of atoms at any instant in the state 1 and 2, respectively. The probability of absorption transition for atoms from state 1 to 2 per unit time is ( ) 12 1 12 P N B u v The probability of transition of atoms from state 2 to 1,either by spontaneously or by stimulated emission per unit time is [ ( )] 21 2 21 21
12 21
In thermal equilibrium at temperature t, the emission and absorption probabilities are equal and thus
1 12 2 21 21
1 12 2 21 2 21 ( ) N B N B
u
1 21 2 21 2 21 ( ) N B N B
u
But Einstein proved thermodynamically that probability of (stimulated) absorption is equal to the probability of stimulated emission, So 12 21
21 1 2 21
3 / 3
h kT e e h u
Planck’s radiation formula gives the energy density of radiation u(v) as (2) from equation (1) and (2) 3 3 21 21
e h B
This equation gives the relation between the probabilities of spontaneous and stimulated emission.
Condition for the laser operation If N 1
N 2