












Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Material Science for Engineers which includes Structure of Wood, Moisture Content, Density of Wood, Mechanical Properties of Wood, Expansion and Contraction of Wood, Concrete Materials, Properties of Concrete etc. Key important points are: Introduction to Materials Science, Classification of Materials, Functional Classification, Materials Based on Structure, Environmental and Effects, Materials Design and Selection, Optical Materials
Typology: Slides
1 / 19

This page cannot be seen from the preview
Don't miss anything!












Section 1.
What is Materials Science and
Engineering?
Section 1.
Classification of Materials
Table 1.1 Continued
© 2003 Brooks/Cole Publishing / Thomson Learning™
Figure 1.4 Representative strengths of various categories of materials
© 2003 Brooks/Cole Publishing / Thomson Learning™
Figure 1.7 Polymerization occurs when small molecules, represented by the circles, combine to produce larger molecules, or polymers. The polymer molecules can have a structure that consists of many chains that are entangled but not connected (thermoplastics) or can form three-dimensional networks in which chains are cross-linked (thermosets)
Figure 1.8 Polymers are used in a variety of electronic devices, including these computer dip switches, where moisture resistance and low conductivity are required. (Courtesy of CTS Corporation.)
Figure 1. Integrated circuits for computers and other electronic devices rely on the unique electrical behavior of semiconducting materials. (Courtesy of Rogers Corporation.)
Figure 1.10 The X- wing for advanced helicopters relies on a material composed of a carbon-fiber- reinforced polymer. (Courtesy of Sikorsky Aircraft Division— United Technologies Corporation.)
© 2003 Brooks/Cole Publishing / Thomson Learning™
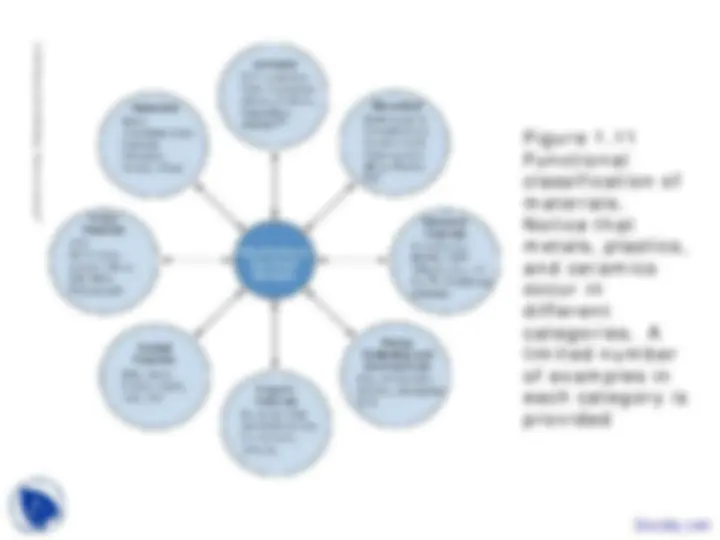
Figure 1. Functional classification of materials. Notice that metals, plastics, and ceramics occur in different categories. A limited number of examples in each category is provided
Section 1.
Classification of Materials-Based on Structure
© 2003 Brooks/Cole Publishing / Thomson Learning™
Figure 1. Increasing temperature normally reduces the strength of a material. Polymers are suitable only at low temperatures. Some composites, special alloys, and ceramics, have excellent properties at high temperatures
© 2003 Brooks/Cole Publishing / Thomson Learning™
Figure 1.13 Skin operating temperatures for aircraft have increased with the development of improved materials. (After M. Steinberg, Scientific American, October, 1986.)
Section 1.
Materials Design and Selection