
















































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Material Science for Engineers which includes Structure of Wood, Moisture Content, Density of Wood, Mechanical Properties of Wood, Expansion and Contraction of Wood, Concrete Materials, Properties of Concrete etc. Key important points are: Heat Treatment, Phase Transformations, Solid-State Reactions, Alloys Strengthened, Precipitation Hardening, Age-Hardened Alloys, Microstructural Evolution, Effects of Aging Temperature
Typology: Slides
1 / 58

This page cannot be seen from the preview
Don't miss anything!



















































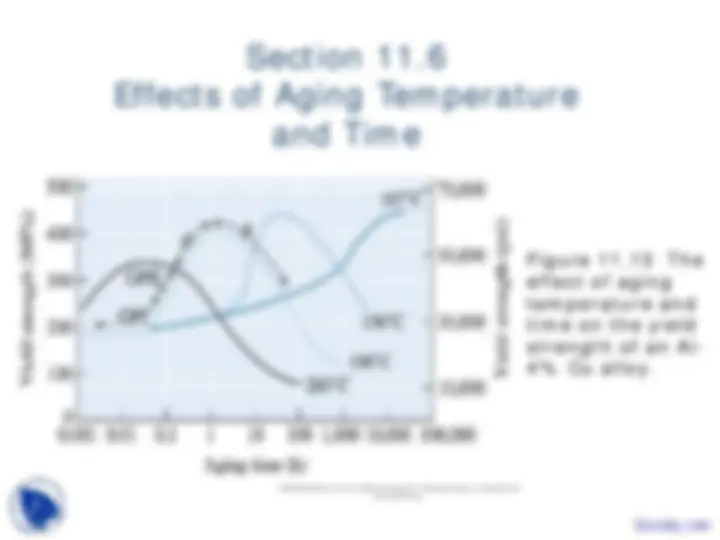
Section 11.
Nucleation and Growth in
Solid-State Reactions
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
Figure 11.2 The effect of temperature on recrystallization of cold-worked copper.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license. Figure 11.3 (a) The effect of temperature on the rate of a phase transformation is the product of the growth rate and nucleation rate contributions, giving a maximum transformation rate at a critical temperature. (b) Consequently, there is a minimum time (t (^) min) required for the transformation, given by the “ C -curve”.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning
is a trademark used herein under license.™
Figure 11. Arrhenius plot of transformation rate versus reciprocal temperature for recrystallization of copper (for Example 11.1.
Section 11.
Alloys Strengthened by
Exceeding the Solubility Limit
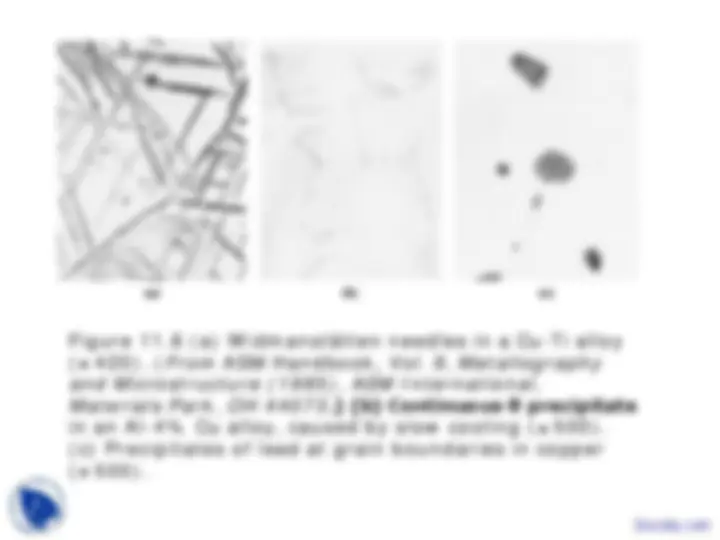
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license. Figure 11.5 The aluminum-copper phase diagram and the microstructures that may develop curing cooling of an Al-4% Cu alloy.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
Figure 11.7 The effect of surface energy and the dihedral angle on the shape of a precipitate.
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license. Figure 11.8 (a) A noncoherent precipitate has no relationship with the crystal structure of the surrounding matrix. (b) A coherent precipitate forms so that there is a definite relationship between the precipitate’s and the matrix’s crystal structure.
Section 11.
Applications of Age-Hardened
Alloys
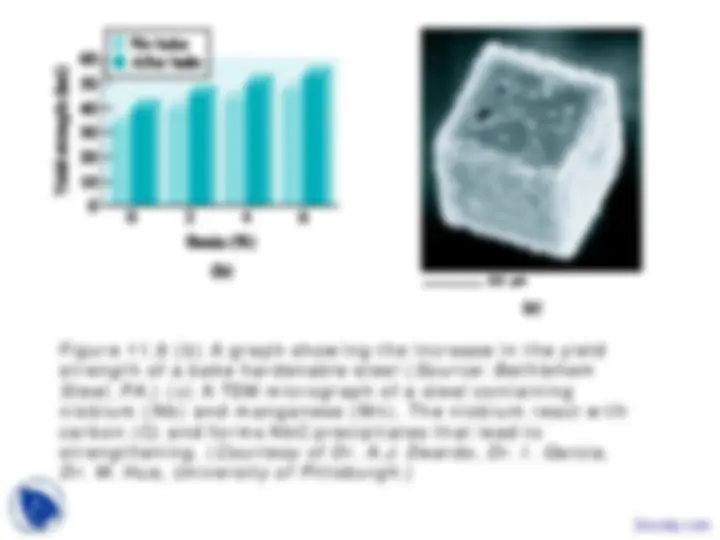
Figure 11.9 (a) A stress-strain curve showing the increase in strength of a bake- hardenable steel as a result of strain hardening and precipitation hardening. ( Source: U.S. Steel Corporation, Pittsburgh, PA .)
Figure 11.9 (b) A graph showing the increase in the yield strength of a bake hardenable steel ( Source: Bethlehem Steel, PA .) (c) A TEM micrograph of a steel containing niobium (Nb) and manganese (Mn). The niobium react with carbon (C) and forms NbC precipitates that lead to strengthening. ( Courtesy of Dr. A.J. Deardo, Dr. I. Garcia, Dr. M. Hua, University of Pittsburgh .)