









Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Science and Engineering of Materials which includes Point Defects, Types of Defects, Equilibrium Number, Thermal Vibrations, Boltzmann Constant, Regular Lattice Sites, Substitutional Solid Solutions, Composition Conversions etc. Key important points are: Growth of Solid Equilibrium, Non-Equilibrium Cooling, Solid Solution Strengthening, Isomorphous Alloys, Binary Eutectic Systems, Eutectic Reaction, Microstructure in Eutectic Alloys, Eutectic Isotherm
Typology: Slides
1 / 17

This page cannot be seen from the preview
Don't miss anything!










Growth of Solid Equilibrium ( = very slow cooling)
In solid + liquid phase region: Solid forms gradually upon cooling from liquidus line
Composition of solid and liquid changes gradually (determine by tie-line method)
At the solidus line: solid nuclei grow to consume all the liquid
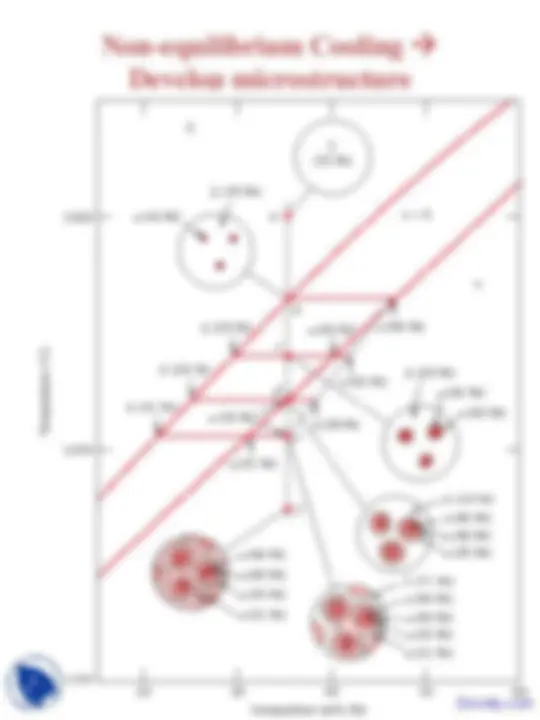
Microstructure in isomorphous alloys Non-equilibrium cooling
(no tie line) ⇒ New layers solidifying on grains have the equilibrium composition at that T ⇒ Formation of layered (cored) grains
Average Ni content of grains is higher?
Application of the lever rule ⇒
Greater proportion of liquid phase as compared to equilibrium at the same T ⇒
Solidus line is shifted to the right ⇒ higher Ni content
Solidification complete at lower T ⇒ Outer part of grains are richer in the low-melting component (Cu).
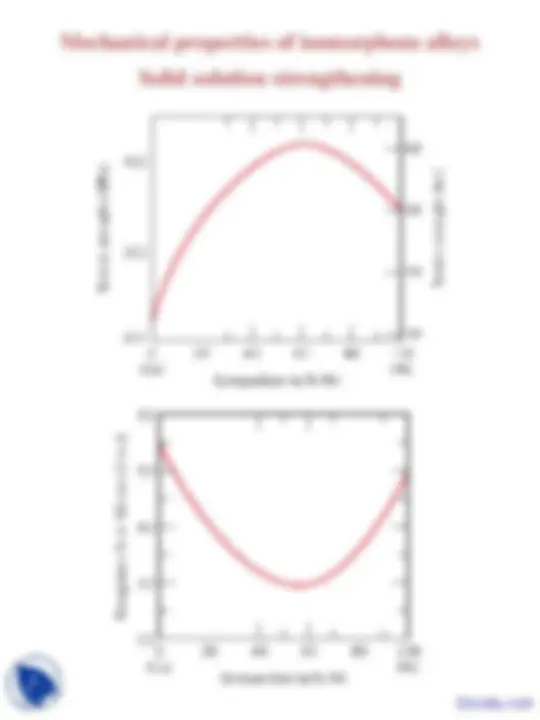
Mechanical properties of isomorphous alloys
Solid solution strengthening
Three single phase regions α - solid solution Ag in Cu matrix, β = solid solution of Cu in Ag matrix, L - liquid
Three two-phase regions (α + L, β +L, α +β)
Solvus separates one solid solution from a mixture of solid solutions. Solvus ⇒ limit of solubility
Copper – Silver phase diagram
Eutectic (invariant) point Liquid + two solid phases co-exist Eutectic composition C (^) E Eutectic temperature TE. Eutectic Isotherm - horizontal solidus line at TE
Lead – Tin phase diagram
Invariant or eutectic point
Eutectic isotherm
Binary Eutectic System
Compositions + relative amounts of phases ⇒ Tie line and lever rule
Microstructure in eutectic alloys
Cooling of liquid lead/tin system at different compositions
Lead-rich alloy (0-2 wt% tin)
Solidification proceeds as for isomorphous alloys
L → α +L → α
No changes above eutectic temperature, T (^) E At T (^) E liquid transforms to α and β phases (eutectic reaction)
L → α + β
Solidification at Eutectic composition
Solidification at Eutectic composition
α and β compositions are very different ⇒ Eutectic reaction ⇒ redistribution of Pb and Sn atoms by diffusion Simultaneous formation of α and β phases ⇒ layered (lamellar) microstructure: eutectic structure
Formation of eutectic structure in lead-tin system. Dark layers are lead-rich α phase. Light layers are the tin-rich β phase.
Microstructure in eutectic alloys Microconstituent – element of microstructure having a distinctive structure. For previous page: microstructure ⇒ two microconstituents: primary α and eutectic structure.
Although the eutectic structure consists of two phases, it is a microconstituent with distinct lamellar structure and fixed ratio of the two phases.
Relative amounts of microconstituents
Treat eutectic as separate phase + apply lever rule
Fractions: primary α phase (18.3 wt% Sn) and eutectic structure (61.9 wt% Sn):
We = P / (P+Q) ; Wα’ = Q / (P+Q)