
































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
It is the study notes and lecture notes needed to score good in green chemistry
Typology: Lecture notes
1 / 39

This page cannot be seen from the preview
Don't miss anything!
































1. Green Chemistry The design of processes that reduce or eliminate the use and production of toxic products is known as green chemistry. The term was first used by Paul T. Anastas in the last decade of the 20th^ century Green Chemistry implies: Prevention of pollution rather than treatment of pollution Environmentally Benign Chemistry Sustainable Chemistry Ecofriendly Chemistry Clean Chemistry Green chemistry should not be confused with environmental chemistry as environmental chemistry deals with various facets of pollution, degree of pollution and treatment of pollution, while as green chemistry does not lead to pollution at all, hence we say it prevents pollution. Thus green chemistry approach is a prevention approach, while as environmental chemistry approach is a treatment approach. Since prevention is better than cure, we may say green chemistry is better than environmental chemistry. To develop a perfectly green chemical pathway is not easy. However green chemists try their best to maximize the greenness in any process as far as possible. Green chemistry is generally aimed at Producing chemicals which are safe for biotic as well as abiotic environment. Using cost and energy effective methods and procedures.
As per Sheldon, E-factor calculated for various industries is depicted below in a tabulated form as:
E-factor, also known as Environmental (Mass) efficiency factor is calculated as: E-factor = Total Waste (kg) / Product (kg)
*The data has been taken from R. A. Sheldon, Chem. & Ind. 1 December 1992, p. 904.
Assignment 1: Work out any two reactions in organic chemistry that produce wastes?
2. Atom Economy: Synthetic methods should be designed to maximize the incorporation of raw material used in to the products. It is one of the fundamental and most important principle of green chemistry. The concept of atom economy has been developed by B.M. Trost. Atom economy is defined as the measure of the amount of reactants that end up directly into the desired product It is often referred to as percent atom utilization. R. A. Sheldon has also developed the same concept given as: % 𝐴𝑡𝑜𝑚 𝐸𝑐𝑜𝑛𝑜𝑚𝑦 = 𝐹𝑜𝑟𝑚𝑢𝑙𝑎𝐹𝑜𝑟𝑚𝑢𝑙𝑎^ 𝑤𝑒𝑖𝑔ℎ𝑡 𝑤𝑒𝑖𝑔ℎ𝑡^ 𝑜𝑓^ 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠^ 𝑢𝑡𝑖𝑙𝑖𝑠𝑒𝑑 𝑢𝑠𝑒𝑑^ 𝑖𝑛^ 𝑡ℎ𝑒 𝑖𝑛^ 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑡ℎ𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛^ 𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠 x 100 % 𝐴𝑡𝑜𝑚 𝐸𝑐𝑜𝑛𝑜𝑚𝑦 = 𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒^ 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟^ 𝑚𝑎𝑠𝑠 𝑚𝑎𝑠𝑠^ 𝑜𝑓 𝑜𝑓^ 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑎𝑙𝑙 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠^ 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 x 100 e.g the percent atom economy of the reaction given below is as:
Type of Industry Annual production (t) E-factor Total Waste (t, approx Oil refining (^106) - 108 ca. 0.1 106 Bulk Chemicals (^104) - 106 < 1- 5 105 Fine Chemicals (^102) - 104 5 - >50 104 Pharmaceuticals (^100) - 102 25 - >100 103
design of safer chemicals. A well-known example of retrometabolic design is that of ethylene glycol, which is used as an antifreeze and has been replaced by propylene glycol which is less hazardous. Ethylene glycol once ingested in to body gets converted in to glyceraldehyde, glyoxylic acid and oxalic acid which are toxic to body, as against propylene glycol which gets metabolized into normal body metabolites like lactic and pyruvic acid. The lethal dose of ethylene glycol for man is 1.4 ml/kg while as the lethal dose of propylene glycol for man is 7 ml/kg. Thus in the design of safer chemicals propylene glycol is used as an antifreeze. [Ref:-Introduction to Green Chemistry 2nd Edition, A. Matlack, CRC Press, Taylor and Francis].
5. Safer Solvents and auxillaries: The use of auxillary substances (solvents, separation agents) should be made unnecessary whenever possible and innocuous when used.
Chemists generally use any organic solvent of their choice in synthetic reactions. Most often these solvents are the volatile organic solvents (VOCs) and have a major environmental concern, as they are able to form low-level ozone and smog through free-radicals air oxidation processes. They are also highly flammable and cause adverse effects like eye-irritation, headaches and allergic skin reactions in human biengs. These facts have made it necessary to use green alternative solvents. However if possible the use of solvents should be avoided. If however there is no choice and use of solvent becomes imperative, it is recommended to use such solvents which are inert, have low
toxicity, easy to recycle without contaminating the products. The solvent selected should not have any negative impact on the environment or human health. e.g. halogenated solvents like (^) CHCl3, CCl 4 are suspected carcinogens and to avoid their use, green alternatives like water, ionic liquids, liquid CO 2 have been used.To avoid the problems associated with the conventional VOCs, immobilized solvents have been harnessed. Such solvents maintain the solvency, are non-volatile and do not pose any environmental problem.
6. Design for energy efficiency: Energy requirements of a reaction should be recognized for their environmental and economic impacts and should be minimized if possible. Wherever possible synthetic methods should be carried out at ambient temperature and pressure. Energy requirements for the chemical reactions must be as minimum as possible Most commonly used conventional energy source in reactions is the thermal energy. Thermal energy is non-specific, as it is not targeted directly at a bond or the molecules undergoing the reaction. Much of the thermal energy is wasted in heating up the reactors, solvents and even general environment. To avoid these things associated with thermal energy, alternate and more specific forms of energy are used. These sources are green energy sources include photochemical, microwave and ultrasonic sources of energy. This principle requires that energy consumption be minimized in the reactions. Various possible ways to improve the energy efficiency in processes include: a) Good insulation and well maintained equipments reduce heat and energy losses.
8. Reduce derivatives: Unnecessary derivatization (blocking groups/ protection/ deprotection/temporary modification of a physical/chemical process should be avoided whenever possible.
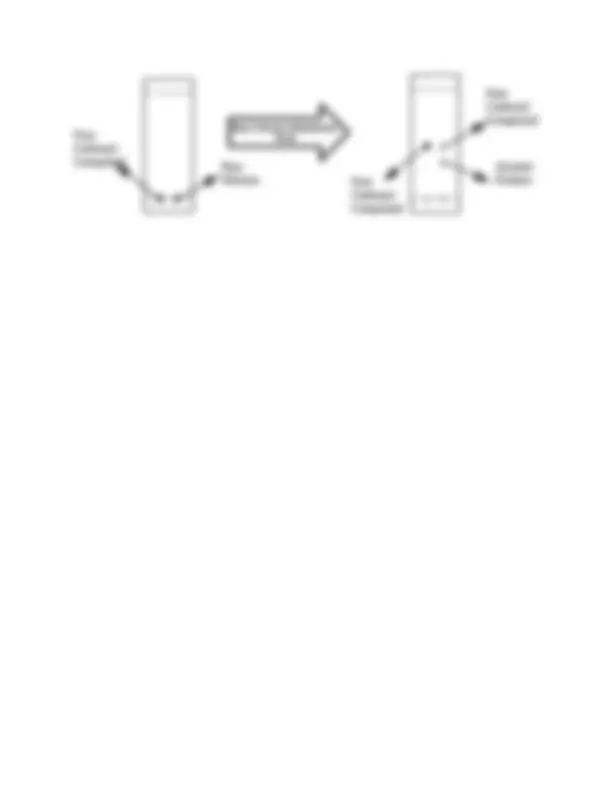
Protecting groups in organic synthesis are important and used to protect a sensitive functionality from reaction reagents/conditions., which make the reaction go away in an unwanted direction, if unprotected. To understand this let us take an example below:
Figure 1: Protection/Deprotection of keto group.
In this example, in order to reduce the ester group in to alcohol group, the ketonic group needs to be protected. Protection is done by using ethylene glycol (II) to get the protected compound (III). The ester moiety in this protected compound can then be reduced by using LiAlH 4 to yield compound (IV). This compound (IV) can then be allowed to undergo acid hydrolysis, i.e. deprotection which yields the desired product with the generation of protecting group, ethylene glycol. Such type of protection and deprotection is too common in organic chemistry. In the above procedure, the protecting group is not incorporated in to the final product, which makes a reaction less atom economical. Thus as far as possible, the use of protecting groups should be avoided.
9. Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents This principle advocates the use of catalytic reagents rather than the stoichiometric reagents. Being unchanged the catalytic reagent can be recovered completely as the reaction comes to an end. All catalysts including enzymes lower the activation energy of reaction, accelerating the reaction several million time, without being changed. Still biocatalysts stand out among all other catalysts because of their specificity in terms of stereochemistry, chemical selectivity. Compared to non-biological catalysts, biocatalysts have a great advantage given the rate of reaction, catalytic specificity, lower cost, etc., but lack of heat sensitivity and poor stability. Biocatalysts are biodegradable-catalysts, which imply less energy consumption. A classical example which demonstrates use of catalytic reagents is furnished by catechol. The classical catechol synthesis is derived from benzene, occurs in a number of steps and involves drastic reaction conditions giving rise to unwanted byproducts. However the biocatalytic route involves synthesis of catechol from glucose in just single step, involves the use of Escherchia coli and is economically viable, involving non formation of byproduct [Ref. Anastas, P. T., Kirchhoff, M. M., Williamson, T. C. (2001): Catalysis as a foundational pillar of green chemistry. Appl Catal A: General, 221: 3-13].
to hydrolysis, photolysis or other possible changes have been used to ensure that products will be biodegradable.
11. Real-time analysis for pollution prevention: Analytical technologies need to be further developed to allow for real time in process monitoring and control prior to the formation of toxic substances.
Analytical technologies need to be developed that will allow the prevention and minimization of generation of hazardous wastes. One need to have accurate and reliable sensors, monitors, and techniques to assess the hazards that are present in the process stream. Using various techniques a process can be monitored for generation of hazardous byproducts and side reactions.
12. Inherently safer chemistry for accident prevention: Substances and the forms of substance used in a chemical process should be choosen to minimize the potential for chemical accidents, including releases, explosions, and fires. This principle requires to reduce the use of such substances in chemical processes that can cause adverse effects (explosion, fire and harmful vapor). Accidents, fire and explosions should be avoided as far as possible. In organic chemistry labs, since solvents find so much of use, it is believed that these vocs can be highly flammable and cause fires. It is to be kept in mind that while doing chemistry, incidents like Bhopal gas tragedy should not happen. 3. Planning a green synthesis
To carry out a green chemical synthesis in a lab, following things are to be considered:
a) Polymer supported per acids:- They are helpful in epoxidation of alkenes. b) Poly NBS:- used as benzylic and allylic brominating agent c) Polymer supported chromic acid:- used in oxidation of alcohols. d) Polystyrene anhydride:- used in acetylation of aniline.
4. Choice of catalysts
Catalysts are versatile chemical entities that increase the rate of reaction without being changed, However not all catalysts are green. Certain metal catalysts are toxic in nature and cause problems both to the biotic as well as abiotic environment. Different types of catalysts have been used and are regarded as green catalysts. These include:-
a) Acid catalysts:- e.g microencapsulated catalysts are replacing the traditional lewis-acid catalysts. Similarly flourided silica alumina catalyst is used in place of the corrosive Hydrogen fluoride. b) Basic catalyst:- Basic catalysts like Mgo and γ-alumina are used in alkylating the benzene rings. c) Oxidation catalysts:-e.g Titanium silicate is used to carry out the hydroxylation of phenol to catechol and resorcinol and quinol. d) Photocatalysts:- A large number of photoatalysts have been used to carry out the transformations. e.g TiO based photo-catalytic systems. e) Biocatalysts: These serve as important tools in green chemistry.
Still biocatalysts stand out among all other catalysts because of their specificity in terms of stereochemistry, chemical selectivity. Compared to non-biological catalysts, biocatalysts have a great advantage given the rate of reaction, catalytic specificity, lower cost, etc., but lack of heat
sensitivity and poor stability. Biocatalysts are biodegradable-catalysts, which imply less energy consumption. e.g daucous carrota enables the reduction of carbonyl groups like aldehydes, ketones, etc, which is a biocatalytic reduction.
Unit 2
1. Designing a green-process
Same as that of planning a green synthesis in a lab. All the sub-headings like choice of starting material, choice of reagents, choice of solvents and choice of catalysts to remain same in this topic as well
2. Prevention of wastes
Normally a synthesis should be carried out in such a way to prevent formation of wastes. If the waste production cannot be ruled out, it is envisaged to carry out a process which could at least minimize the amount of waste generated. Because once the waste is produced it needs to be disposed of. This leads to overall cost of the process. Further the process should be carried out to ensure 100 percent utilization of the reactants, because any amount of starting material that remains unutilized gets mixed with the products and is regarded as waste. Finally anything that is produced in a laboratory ends as a waste. Because once the product is formed, it is put to use and ultimately is to be discarded and thus ends up as a waste. These wastes can pose any serious problems to the biotic and abiotic environment. To prevent this, the waste minimization, recycling and reuse of wastes is actively employed. Most commonly it is envisaged to reduce the minimization of waste at the source of its production. If however reduction of waste at source cannot be avoided, we should try to reuse or recycle the waste to recover material and or energy. Disposal of waste should be the last choice only. This can be shown below:
INCREASING GREENNESS Reduction at source Reuse Recycle, Energy RecoveryR Disposal of waste
3. Maximum incorporation of reactants in to products (Atom economy)
It is one of the fundamental and most important principle of green chemistry. The concept of atom economy has been developed by B.M. Trost. Atom economy is defined as the measure of the amount of reactants that end up directly into the desired product. It is often referred to as percent atom utilization. It gives us the index of how much reactants end up as desired products and how many end up as waste.
R. A. Sheldon has also developed the same concept given as:
% 𝐴𝑡𝑜𝑚 𝐸𝑐𝑜𝑛𝑜𝑚𝑦 = 𝐹𝑜𝑟𝑚𝑢𝑙𝑎𝐹𝑜𝑟𝑚𝑢𝑙𝑎^ 𝑤𝑒𝑖𝑔ℎ𝑡 𝑤𝑒𝑖𝑔ℎ𝑡^ 𝑜𝑓^ 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠^ 𝑢𝑡𝑖𝑙𝑖𝑠𝑒𝑑 𝑢𝑠𝑒𝑑^ 𝑖𝑛^ 𝑡ℎ𝑒 𝑖𝑛^ 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑡ℎ𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛^ 𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠 x 100 𝑜𝑟 % 𝐴𝑡𝑜𝑚 𝐸𝑐𝑜𝑛𝑜𝑚𝑦 = 𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒^ 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟^ 𝑚𝑎𝑠𝑠 𝑚𝑎𝑠𝑠^ 𝑜𝑓 𝑜𝑓^ 𝑑𝑒𝑠𝑖𝑟𝑒𝑑 𝑎𝑙𝑙 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠^ 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 x 100 Or % 𝐴𝑡𝑜𝑚 𝐸𝑐𝑜𝑛𝑜𝑚𝑦 = (^) 𝑀𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟 𝑤𝑖𝑒𝑔ℎ𝑡 𝑜𝑓 𝑎𝑙𝑙 𝑡ℎ𝑒 𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠𝑀𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟 𝑚𝑎𝑠𝑠^ 𝑜𝑓^ 𝑑𝑒𝑠𝑖𝑟𝑒𝑑^ 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 x 100
Calculation of atom economy
For a general reaction:
A + B C
% Atom economy is given by
% 𝐴𝑡𝑜𝑚 𝐸𝑐𝑜𝑛𝑜𝑚𝑦 = (^) 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡 𝐴+𝑀𝑎𝑠𝑠 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡 𝐵𝑀𝑎𝑠𝑠 𝑜𝑓 𝑃𝑟𝑜𝑑𝑢𝑐𝑡 𝐶 x 100
Similarly