























Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Question related to GOC for comptetive exams such as IIT JEE MAINS, ADVANCE AND NEET
Typology: Assignments
1 / 30

This page cannot be seen from the preview
Don't miss anything!























1. Determine the inductive effect of phenyl ring on attached group in the (a) and (b) molecules respectively
O
(a) (b) (a) a : + I , b : + I (b) a : – I , b : + I (c) a : + I , b : – I (d) a : – I , b : – I
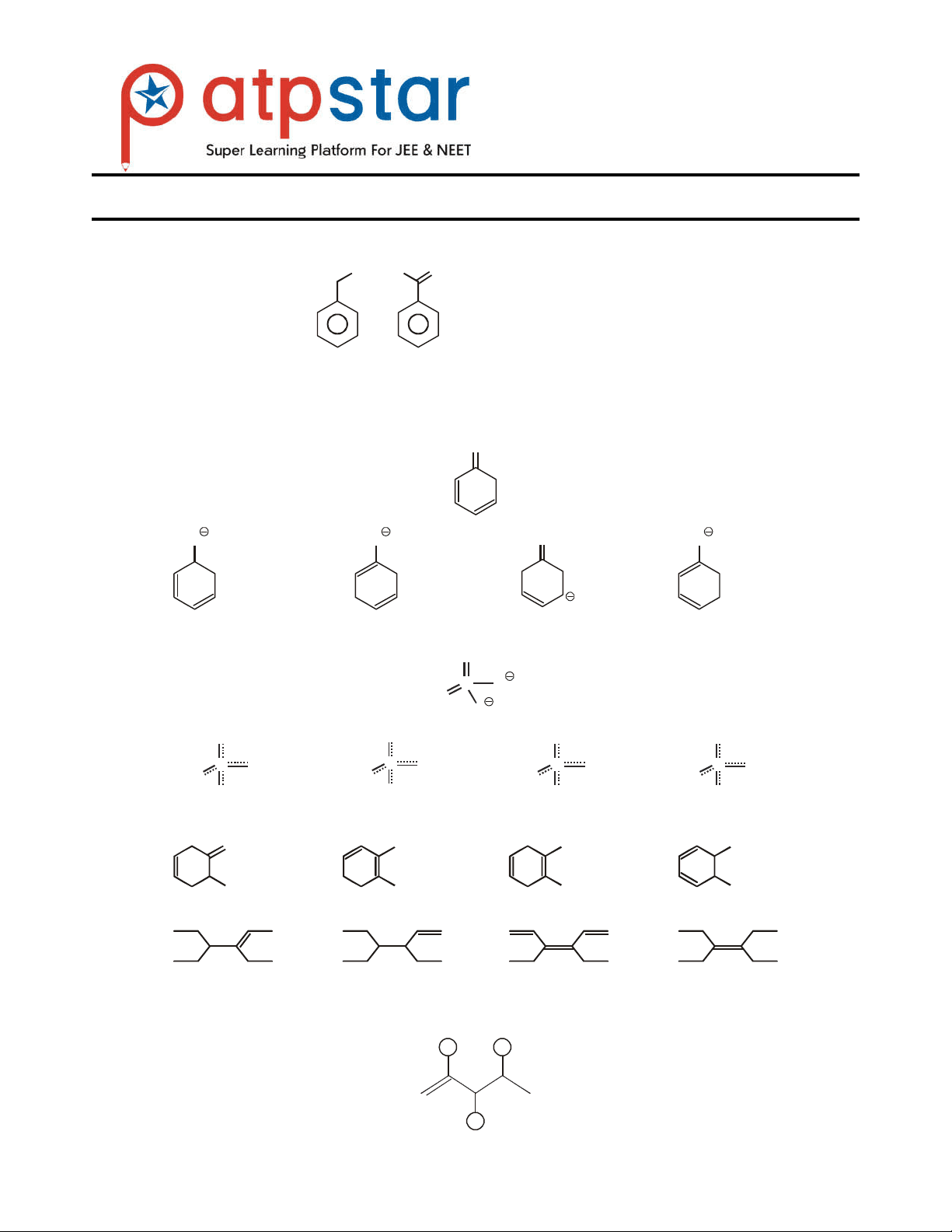
2. Which is the least stable resonating structure of the given molecule? O
(a)
O (b)
O
(c)
O
(d)
O
3. Identify the resonance hybrid of the given ion :
S
O O O O
(a) S
O –1/ O –1/ O –1/
–1/4 (^) O (b) S
O –1/ O –1/ O –1/
–1/2 (^) O (c) S
O –3/ O^ –3/ O –3/
–3/4 O (d) S
O – O – O –
–1 O
4. Which of the following is the most stable?
(a) (b) (c) (d)
5. Which of the following has minimum heat of hydrogenation?
(a) (b) (c) (d)
6. The decreasing order of bond dissociation energies amongst the C–H bonds labelled x, y, z (in homolysis) is: H z (^) x
y
H
H (a) x > y > z (b) x > z > y (c) z > x > y (d) y > z > x
Rank Boosting Course (RBC)
7. Determine the stability order of given intermediates.
Me OMe
Me (I)
OMe
(II)
Me (^) (^) OMe
(III)
H C 2 H C — OMe 2
(IV)
(a) I > II > III > IV (b) I > II > IV > III (c) IV > I > II > III (d) III > I > II > IV
8. Rank the following reactions in DECREASING order of heat of hydrogenation.
(a)
2H (^2) (b)
2H (^2)
(c)
2H (^2)
(a) a>c>b (b) c>a>b (c) a>b>c (d) b>c>a
9 Which of the following bonds is the longest?
O
NH (^2)
(a) NH (^2)
(b) CH = NH 2
(c) NH (^2)
(d)
(a) a (b) b (c) c (d) d
10. What is the correct order of stability among the given carbocations?
CH 2
CH 2
(I) (II) (III) (IV) (a) II > I > IV > III (b) II > I > III > IV (c) I > II > IV > III (d) I > II > III > IV
11. Which of the following are not resonating structures of each other? (a) CH 3 – N = C = S & CH 3 – S – C N
(b) CH 3 C O
& CH^3 C^ O
(c) (^) CH –C–OH & CH –C=O–H 3 3
O O
(d) CH 2 = CH – C N & CH –CH=C=N 2
12. Find correct order of resonance energy for
(I) (^) OCH 3 (II) OCH (^3)
(a) III > I > II (b) II > I > III (c) I > II > III (d) III > II > I
13. Which of the following substituents will decrease the acidic strength of phenol? (a) – NO 2 (b) – CN (c) – CH 3 (d) – CHO
23. In which of the following, the 2nd structure has more resonance energy.
(a) O
vs N H
(b) vs
CH 3
(c) CH 2 =CH–CH=CH– CH^2 vs (d) All
24. Which compound has identical C–C bond length?
(a) (b) (c) O
(d)
25. Which compound is most basic among the following?
(a) I (b) II (c) III (d) IV
26. What is bond length order? a (^) b c d (a) d > b > a = c (b) d > a = b = c (c) b = d > a = c (d) d < a = b = c 27. Increasing order of stability of following resonating structures is :
N N N
O
C C
(I) (II) (III)
C
O O (a) I < II < III (b) III < II < I (c) II < III < I (d) II < I < III
28. Which group has greater +M effect than —N?
(a) –NH 2 (b) –NH–C–Me O
(c) (^) O (d) –OH
29. Which of the following will have lowest dissociation constant (Ka)?
(a) COOH (b) COOH
NO 2
(c) COOH^ (d) COOH
Cl
30. Select the one which is most basic among the following:
(a) N–H^ (b) N
Me Me
(c) O^ N–H (d) O
31. Which of the following pair having same functional group?
(a) CH 3 –CH 2 – NH 2 , CH – CH – NH 3 2
CH (^3)
(b)
OH OH
(c) CH – C – O – CH 3 3
O
(d) (^) CH – 3 CH – 2 C – H
O
32. Which of the following is not + I group? (a) –SiMe 3 (b) –CMe 3 (c) –COO¯ (d) None of these 33. The correct order of decreasing basicity of the compounds is:
Et
Et NEt (II) N
N
N H (a) I > II > III > IV (b) III > I > II > IV (c) I > III > II > IV (d) I > III > IV > II
34. Which compound has lesser C–O bond length in comparison to C–O bond length in
O
(a)
O
(b)
OH
(c)
O
(d)
O
N
H
(c) –Ph < NO^2 < NH 2
(d) (^) C NH C 2 H 5
<^ C OCH 3
<^ C H
41. 1 mol of which compound can release maximum heat when it reacts with air? (a) E-cyclo decene (b) Z–cyclo decene (c) E–cyclo pentadecene (d) Z– cyclo pentadecene 42. Which of the following is a +I group?
(a) –C–H
O (b) –C–Me
O (c) –C–NH (^2)
O (d) (^) –C–O
O
43. Which of the following statement is correct? (a) The relative order of + I groups is –O > NH > –CH (^2) (b) The relative order of – I groups is - NF 3
NMe 3
(c) The relative order of basic strength in aqueous solution is NH 3 < MeNH 2 < Me 2 NH < Me 3 N (d) None of these
44. Which of the following compound is least basic?
(a) N H
(b) N H
O
(c) (^) N H
O
(d) (^) N H
O O
45. Which of the following is least stable carbanion?
(a) CH 3 OCH 3 (b)
OCH 2
NO 2
(c)
OCH 2
CN
(d)
OCH 2
OCH 3
46. Arrange the following in decreasing order of acidic strength.
COOH
(II)
COOH
NO 2
COOH
SO H 3
COOH
CH 3 (a) I > II > III > IV (b) II > III > IV > I (c) III > II > I > IV (d) III > I > II > IV
47. Find the number of sp^2 hybridised atoms in the following compound.
C N
CH=CH–CH=CH 2 (a) 12 (b) 14 (c) 15 (d) 11
48. Which of the following resonating structure is most stable?
(a) CH 3 (^) O CHCHCH 2 (b)
CH 3 (^) O CHCHCH 2
(c) CH 3 O^ CH^ CH^ CH 2
(^) (d) CH 3 OCHCHCH 2
49. Which of the following can show resonance with the alkene?
(a) (b)
O
(c)
CN (d)
COOH
50. Which of the following is non-aromatic?
(a) (b) N
(c) (d)
51. In the following compounds:
(I)
OH OH
CH 3 (II)
NO 2
OH
(III)
OH
NO 2 (IV)
The order of acidity is: (a) III > IV > I > II (b) I > IV > III > II (c) II > I > III > IV (d) IV > III > I > II
52. Which of the following compound is most basic?
(a) CH – C – NH 3 2
O (b) (^) H 2 N– C – NH 2
NH (c) (^) CH – C – NH 3 2
NH (d) Ph – C – NH 2
O
53. Select the incorrect statement for
(a) 4 electrons are participating in resonance (b) Compound is anti aromatic (c) It is a Homocyclic compound (d) Compound is non aromatic
62. Which of the following compound has least value of pKa?
(a) O
OH (b) O OH
(c) O (^) OH
(d) O
63. In which pairs, specie-I is more stable than specie-II?
(a) H–C–CH –O 2
O , CH –C–O 3
O
(b) (^) CH –C–CH–CH –C–CH , CH –C–CH–C–CH 3 2 3 3 3
O O O O
(c) CH –CH–CH –C–CH , CH –CH –CH–C–CH 3 2 3 3 2 3
O O
(d) N
64. Which of the following is least acidic compound?
(a)
COOH
(b)
OH
(c)
OH
NO 2
(d)
SH
65. Which resonating structure is most stable for
CH 2
N O O
(a)
CH 2
N O O
(b)
CH 2
N O O
(c)
CH 2
N O O
(d)
CH 2
N O O
66. Which of the following alkene is least stable?
(a) (b) (c) (d)
67. Heat of combustion is be maximum for
(a) (b) (c) (d)
68. Which of the following has the highest heat of hydrogenation?
(a) (b) (c) (d)
69. Which of the following carbanion is most stable?
(a)
O
(b)
O
(c)
O
(d)
O
(A)
H 2
^1 ^ mole
Which isomer of A has maximum heat of combustion?
(a) (b) (c) (d)
71. Which of the following pairs are resonance structures of each other?
(I)
O
H C 3 CH 3
OH
H C 3 CH 2
C
(II)
(III)
(^) (IV) (a) I, II, III, IV (b) I, IV (c) I, II, III (d) I, III, IV
72. ‘y’ = Number of compound having –I group directly attached to benzene. SO H 3 CN COOH NO 2
CH 3 CF 3 OH
Find the value of ‘y’? (a) 5 (b) 6 (c) 7 (d) 8
73. Which of the following is not an aromatic compound?
(a) (^) S (b)
O
B H
(c) (d)
80. Determine the correct order of acidity of the following compounds.
O
O
O
O
O
O
HO
HO (i) (ii) (iii) (a) i > ii > iii (b) ii > iii > i (c) iii > i > ii (d) iii > ii > i
81. Which of the following is most stable resonance structure?
(a)
2
2
(b)
2
2
(c)
2
2
(d)
2
2
82. Compare rotational energy barrier about indicated C = C between given compounds.
n-C H 3 7
n-C H 3 7 (II) n-C H 3 7
n-C H 3 7
CHO
n-C H 3 7
n-C H 3 7
CHO
CHO
(a) I > II > III (b) III > II > I (c) III > I > II (d) I = II = III
83. Which of the following is correct order of – I?
(a) –F > – NO 2 > – CN > Br (b) –NH > NO > –CN > –C–H 3 2 O (c) (^) –NH > –NH Me > – NHMe > – NMe 3 2 2 3 (d) (^) –NH > – OH > –Cl > –Br 2
84. Compare bond length of the indicated bond.
(i) (^) (ii)
NH–Me (iii) (iv) C N
NH–Me
(a) (i) > (ii) > (iii) > (iv) (b) (iv) > (iii) > (ii) > (i) (c) (iv) > (ii) > (iii) > (i) (d) (i) > (iii) > (ii) > (iv)
85. Which amongst the following has the most extensive hydrogen bonding in aqueous medium? (a) Ethanol (b) Acetaldehyde (c) Acetone (d) Acetic acid 86. Compare acidic strength among the given compounds.
H H
Cl Cl
COOH
H H
Cl Cl
COOH (a) I > II (b) II > I (c) I = II (d) Can not be compared
87. Which of the order is incorrect?
(a) Acidic strength : O – C – C – OH O O
O O
(b) Acidic strength : F
F F
F
F C –OH O
O
(c) Stability order : CH 3
N CH 3 CH 3
N N
(d) Basic Strength order : Et 2 NH > Et 3 N > Et–NH 2 > NH 3 (In H 2 O)
88. The correct order of basic strength for the following bases is / are :
(a) CH 3 > NH 2 > OH > F
(b)
NH 2 NH 2 NH (^2) CH 3
CH 3 CH 3 (c) Both are correct (d) Both are incorrect
89. Which of the following has highest heat of hydrogenation?
(a) (b)
(c) (d)
OH
OH
HOOC SO 3 H
NO 2
NaNH ^2
2 moles X
The product X will be
(a)
OH
HOOC SO^3
O
NO 2
(b)
OH
OOC SO^3
OH
NO 2
(c)
OH
OOC SO 3 H
O
NO 2
(d) (^) OOC SO (^3)
O
O
NO 2
96. Identify the compound having highest basic strength.
(a)
NH
NH 2
OCH 3
(b)
NH
H 2 N NH
CHO
(c) H 2 N NH
NH 2 (d) H 2 N NH
CH 3
97. Which of the following compounds will have highest thermodynamic acidic strength?
(a)
SO H 3
(b) p-nitro phenol
(c) p-Sulpho Benzoic acid (d) 2,4-Dinitro phenol
98. Arrange the compounds in decreasing acidic strength order:
O (^) O || (^) || CH C CH C CH (II)^3
O || CH C CH (III) CHCH (IV) CH 3 –CHO (a) I > IV> III > II (b) I > IV > II >III (c) III > I > IV > II (d) II > IV > I > III
99. The correct basic strength order of the following bases is:
CH (^3)
O
NH (^) C (II)
C (^) NH CH 3
O
C (^) CH NH 2 2
O
C (^) CH 3
O H 2 N
(a) I > II > IV > III (b) IV > III > II > I (c) III > II > IV > I (d) III > IV > II > I
100. Which of the following hydrocarbon is most acidic?
(a) (b) (c) (d)
10. (c)
Sol. Carbocation stability :
( -bond resonance) (Dancing resonance) (Aryl carbocation)
>>
(unstable)
vacant sp^2
(5RS)
(2RS)
11. (a) Sol. First pair is not resonating structure because the position of atoms is not same. 12. (c) Sol. The order of resonance energy is I > II > III greater the resonance, greater will be resonance energy and thus III has the lowest resonance energy then I(Extended resonance) > II(cross resonance). 13. (c) Sol. Acidic strength of phenol will decrease when electron releasing group will be substituted. 14. (d) Sol. Greater the number of hyperconjugation greater will be the stability of carbocation. 15. (a)
Sol. is an aromatic compound since it is cylic planar, conjugated system and follow Huckel’s rule. It
has (4n + 2) - electron where n = 1
16. (b) Sol. Structure can not be changed in resonance. 17. (b)
Sol. H C–CH –C–O^3 O
CH –CH –C=O 3 2 O
Bond order = Number of bonds between atoms 3 Resonating structures 2
18. (b) Sol. More the hydroxy groups, more is H-bonding with solvent water and thus more is the solubility.
19. (c)
Sol. HOHhyd. (^) stability
20. (a) Sol. (I) 9 H (II) 9 D Bond strength order : C – D > C–H +H order : C – D < C–H 21. (d)
Sol. H
H H
CH (^2)
H
H H
H
H
H
H
H
Hyperconjugation structures of isobutene and trans-2- butene respectively have 1° anion and 2° anion, 1° anion is more stable than 2° anion.
22. (c)
Sol. more -hydrogen, more will be the hyperconjugation, more will be the [C=C] bond length.i.e.
it will have more single bond character
23. (d) Sol. In (a) N is better donor than O due to lesser electronegativity. In (b) and (c), the second compound is aromatic. 24. (d)
Sol. Compound has identical C – C bond length as the negative charge participates in resonance
and molecule is aromatic.