























































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These notes may be used freely by A level biology students and teachers, and they may be copied and edited. Please do not use these materials for commercial ...
Typology: Summaries
1 / 66

This page cannot be seen from the preview
Don't miss anything!



























































Specification Biological Molecules Water Carbohydrates Lipids Proteins Enzymes DNA DNA Gene Expression Gene Mutations Viruses Viruses Viral Diseases Cell Division Cell cycle and Mitosis Meiosis Chromosome Mutations Sexual Reproduction Sexual reproduction in Mammals Sexual reproduction in Plants Classification and Evolution Classification Natural Selection Speciation Biodiversity
These notes may be used freely by A level biology students and teachers, and they may be copied and edited. Please do not use these materials for commercial purposes. I would be interested to hear of any comments and corrections. Neil C Millar (nmillar@ntlworld.co.uk) Head of Biology, Heckmondwike Grammar School High Street, Heckmondwike, WF16 0AH July 2015
1.01 Water The importance of the dipole nature of water leading to hydrogen bonding and the significance of the following to organisms: high specific heat capacity; polar solvent; surface tension; incompressibility; maximum density at 4 °C.
1.02 Carbohydrates. The difference between monosaccharides, disaccharides and polysaccharides. The structure of the hexose glucose (alpha and beta) and the pentose ribose. How monosaccharides (glucose, fructose, galactose) join to form disaccharides (sucrose, lactose and maltose) and polysaccharides (starch formed from amylose and amylopectin; glycogen) through condensation reactions forming glycosidic bonds, and how these can be split through hydrolysis reactions. How the structure of glucose, starch, glycogen and cellulose relates to their function.
1.03 Lipids How a triglyceride is synthesised, including the formation of ester bonds during condensation reactions between glycerol and three fatty acids. The differences between saturated and unsaturated lipids. How the structure of lipids relates to their role in energy storage, waterproofing and insulation. How the structure and properties of phospholipids relate to their function in cell membranes.
1.04 Proteins The structure of an amino acid (structures of specific amino acids are not required). The formation of polypeptides and proteins (as amino acid monomers linked by peptide bonds in condensation reactions). The role of ionic, hydrogen and disulphide bonding in the structure of proteins. The significance of the primary, secondary, tertiary and quaternary structure of a protein in determining the properties of fibrous and globular proteins, including collagen and haemoglobin. How the structure of collagen and haemoglobin are related to their function.
1.05 Enzymes Enzymes are catalysts that reduce activation energy. Enzymes catalyse a wide range of intracellular reactions as well as extracellular ones. The structure of enzymes as globular proteins. The concepts of specificity and the induced fit hypothesis.
How the initial rate of enzyme activity can be measured and why this is important. Temperature, pH, substrate and enzyme concentration affect the rate of enzyme activity. Enzymes can be affected by competitive, non-competitive and end-product inhibition.
1.06 DNA
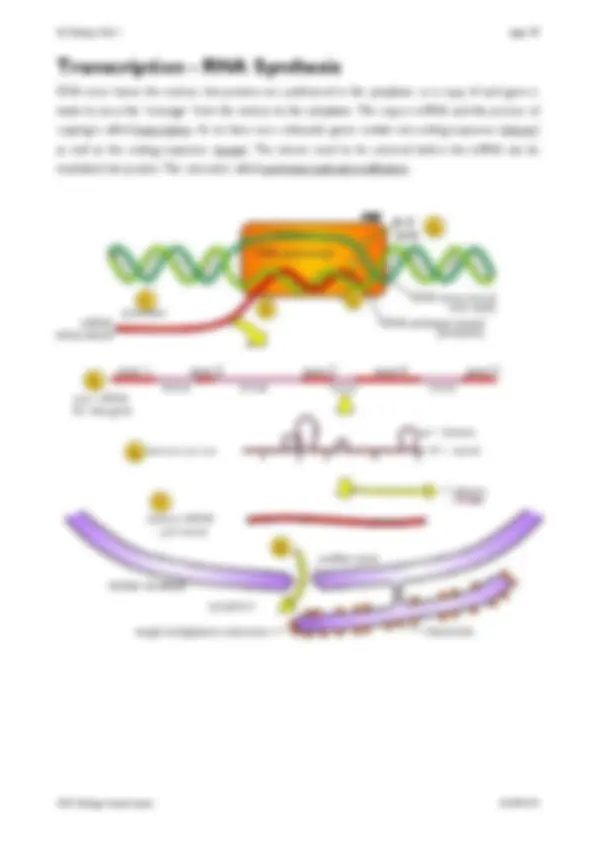
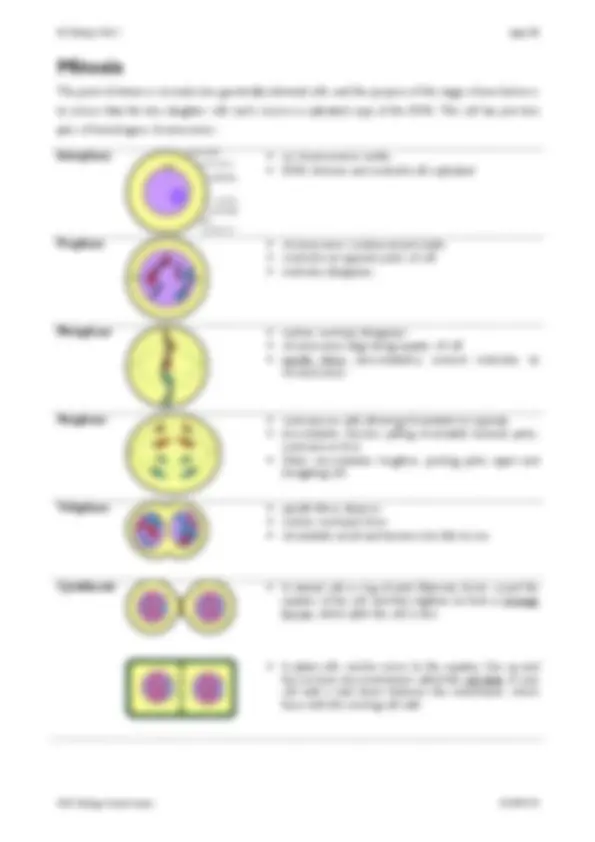
The structure of DNA, including the structure of the nucleotides (purines and pyrimidines), base pairing, the two sugar-phosphate backbones, phosphodiester bonds and hydrogen bonds. How DNA is replicated semi-conservatively, including the role of DNA helicase, polymerase and ligase. 1.07 Gene Expression A gene is a sequence of bases on a DNA molecule coding for a sequence of amino acids in a polypeptide chain. The structure of mRNA including nucleotides, the sugar phosphate backbone and the role of hydrogen bonds. The structure of tRNA, including nucleotides, the role of hydrogen bonds and the anticodon. The nature of the genetic code, including triplets coding for amino acids, start and stop codons, degenerate and non-overlapping nature, and that not all the genome codes for proteins. The processes of transcription in the nucleus and translation at the ribosome, including the role of sense and anti-sense DNA, mRNA, tRNA and the ribosomes. 1.08 Gene Mutations The term gene mutation as illustrated by base deletions, insertions and substitutions. The effect of point mutations on amino acid sequences, as illustrated by sickle cell anaemia in humans. 1.09 Viruses The classification of viruses is based on structure and nucleic acid types as illustrated by λ (lambda) phage (DNA), tobacco mosaic virus and Ebola (RNA) and human immunodeficiency virus (RNA retrovirus). The lytic cycle of a virus and latency. 1.10 Viral Diseases Viruses are not living cells and so antivirals must work by inhibiting virus replication. as viruses can be difficult to treat once infection has occurred, the focus of disease control should be on preventing the spread, as exemplified by the 2014 Ebola outbreak in West Africa. Be able to evaluate the ethical implications of using untested drugs during epidemics. 1.11 Cell Cycle and Mitosis The cell cycle is a regulated process in which cells divide into two identical daughter cells, and that this process consists of three main stages: interphase, mitosis and cytokinesis. What happens to genetic material during the cell cycle, including the stages of mitosis. Mitosis contributes to growth, repair and asexual reproduction. 1.12 Meiosis Meiosis results in haploid gametes, including the stages of meiosis. Meiosis results in genetic variation through
Living things are made up of thousands and thousands of different chemicals. These chemicals are called organic because they contain the element carbon. In science organic compounds contain carbon–carbon bonds, while inorganic compounds don’t. There are four important types of organic molecules found in living organisms: carbohydrates, lipids, proteins, and nucleic acids (DNA). These molecules are mostly polymers, very large molecules made up from very many small molecules, called monomers. Between them these four groups make up 93% of the dry mass of living organisms, the remaining 7% comprising small organic molecules (like vitamins) and inorganic ions.
Group name Elements Monomers Polymers % dry mass of a cell Carbohydrates CHO monosaccharides polysaccharides 15 Lipids CHOP fatty acids + glycerol* triglycerides* 10 Proteins CHONS amino acids polypeptides 50 Nucleic acids CHONP nucleotides polynucleotides 18
We'll study each of these groups in turn.
In biochemistry there are three important types of chemical bond. Covalent bonds are strong. They are the main bonds holding the atoms together in the organic molecules in living organisms. Because they are strong, covalent bonds don’t break or form spontaneously at the temperatures found in living cells. So in biology covalent bonds are always made or broken by the action of enzymes. Covalent bonds are represented by solid lines in chemical structures.
Ionic Bonds are fairly strong. They are formed between a positive ion (such as
ionic compounds dissociate in solution, but ionic bonds are sometimes found inside protein molecules.
Hydrogen bonds are much weaker. They are formed between an atom (usually hydrogen) with a slight positive charge (denoted +) and an atom (usually oxygen or nitrogen) with a slight negative charge (denoted –). Because hydrogen bonds are weak they can break and form spontaneously at the temperatures found in living cells without needing enzymes. Hydrogen bonds are represented by dotted lines in chemical structures.
must lose a lot of heat to change state from a liquid to a solid. This means it is difficult to freeze water, so ice crystals are less likely to form inside cells.
4. Water is cohesive and adhesive. Cohesion means that water molecules "stick together" due to their hydrogen bonds. This explains why long columns of water can be sucked up tall trees by transpiration without breaking. It also explains surface tension, which allows small animals to walk on water. Adhesion means that water molecules stick to other surfaces, such as xylem vessels. This explains capillary action (where water will be drawn along a narrow tube) and the meniscus on test tube walls. 5. Water is most dense at 4°C. Most substances get denser as they cool down, and the solid form is denser than the liquid form. Water is unique in that the solid state (ice) is less dense that the liquid state, and in fact water is most dense at 4°C. This property causes several important effects:
Ice floats on water, so as the air temperature cools, bodies of water freeze from the surface, forming a layer of ice with liquid water underneath. This allows aquatic ecosystems to exist in sub-zero temperatures, and even throughout long ice ages. The expansion of water as it freezes causes freeze-thaw erosion of rocks, which results in the formation of soil, without which there could be no terrestrial plant life. Cold water sinks below warm water, and warm water rises above cold water, which gives rise to many ocean currents.
6. Water is incompressible. The hydrogen bonds hold water molecules closer together than other liquids, so water is very incompressible, since the molecules can’t be pushed any closer. So if a force is applied to water, the water will move rather than squash, which allows blood to be pumped round a body. The incompressibility is also used to make plant cells turgid and give eyes their shape.
Carbohydrates
Carbohydrates contain only the elements carbon, hydrogen and oxygen. The group includes monomers, dimers and polymers, as shown in this diagram:
Monosaccharides Monosaccharides all have the formula (CH 2 O)n, where n can be 3-7. Hexose sugars have six carbon atoms, so have the formula C 6 H 12 O 6. Hexose sugars include glucose, galactose and fructose. These are isomers, with the same chemical formula (C 6 H 12 O 6 ), but different structural formulae. In animals glucose is the main transport sugar in the blood, and its concentration in the blood is carefully controlled. Pentose sugars have five carbon atoms, so have the formula C 5 H 10 O 5. Pentose sugars include ribose and deoxyribose (found in nucleic acids and ATP) and ribulose (which occurs in photosynthesis). Triose sugars have three carbon atoms, so have the formula C 3 H 6 O 3. Triose sugars are found in respiration and photosynthesis.
Structural formula for - Glucose (C 6 H 12 O 6 )
You need to know these formulae!
Structural formula for Ribose (C 5 H 10 O 5 )
Polysaccharides Polysaccharides are chains of many glucose monomers (often 1000s) joined together by glycosidic bonds. The main polysaccharides are starch, glycogen and cellulose.
Amylopectin is poly(1-4) glucose with about 4% (1-6) branches. This gives it a more open molecular structure than amylose. Because it has more ends, it can be broken more quickly than amylose by amylase enzymes. Both amylose and amylopectin are broken down by the enzyme amylase into maltose, though at different rates.
This apparently tiny difference makes a huge difference in structure and properties. The bond is flexible so starch molecules can coil up, but the bond is rigid, so cellulose molecules form straight chains. Hundreds of these chains are linked together by hydrogen bonds between the chains to form cellulose microfibrils. These microfibrils are very strong and rigid, and give strength to plant cells, and therefore to young plants and also to materials such as paper, cotton and sellotape.
The -glycosidic bond cannot be broken by amylase, but requires a specific cellulase enzyme. The only organisms that possess a cellulase enzyme are bacteria, so herbivorous animals, like cows and termites whose diet is mainly cellulose, have mutualistic bacteria in their guts so that they can digest cellulose. Carnivores and omnivores cannot digest cellulose, and in humans it is referred to as fibre.
Starch and Glycogen Cellulose glycosidic bonds glycosidic bonds flexible chains straight chains H bonds within each chain, forming helix H bonds between chains, forming microfibrils Can form H-bonds with water, so can be soluble Can't form H bonds with water, so insoluble Reacts with iodine to form blue-black complex Doesn't react with iodine Easy to digest Difficult to digest Storage role Structural role
Triglycerides are found in fatty (or adipose) tissue. They are used for: Energy storage. Triglyceride respiration yields more energy per unit mass than other compounds, so adipose tissue is used a long-term energy store. However, triglycerides can't be mobilised quickly since they are so insoluble, so are no good for quick energy requirements. Tissues that need energy quickly (like muscles) instead use glycogen. Insulation. Adipose tissue under the skin (sub-cutaneous) is used to insulate warm-blooded mammals against heat loss e.g. blubber in whales. A fatty myelin sheath electrically insulates nerve cells so the electrical impulses travel faster. Waterproofing. Mammals’ fur and birds’ feathers contain the lipid lanolin for waterproofing. Insect exoskeletons contain waxy lipids to stop water loss, and plants have a lipid waxy cuticle to reduce water loss.
Saturated and Unsaturated Fats If the fatty acid chains in a triglyceride have no C=C double bonds, then they are called saturated fatty acids (i.e. saturated with hydrogen). Triglycerides with saturated fatty acids have a high melting point and tend to be found in warm-blooded animals. At room temperature they are solids (fats), e.g. butter, lard. If the fatty acid chains in a triglyceride do have C=C double bonds they are called unsaturated fatty acids (i.e. unsaturated with hydrogen). Fatty acids with more than one double bond are called poly-unsaturated fatty acids (PUFAs). Triglycerides with unsaturated fatty acids have a low melting point and tend to be found in cold-blooded animals and plants. At room temperature they are liquids (oils), e.g. fish oil, vegetable oils. An “omega number” is sometimes used to denote the position of a double bond, e.g. omega-3 fatty acids.
saturated
unsaturated
Phospholipids Lipids have a very low density, so the body fat of water mammals helps them to float easily Phospholipids have a similar structure to triglycerides, but with a phosphate group in place of one fatty acid chain. There may also be other groups attached to the phosphate. Phospholipids have a polar hydrophilic "head" (the negatively-charged phosphate group) and two non-polar hydrophobic "tails" (the fatty acid chains).
or
This mixture of properties is fundamental to biology, for phospholipids are the main components of cell membranes. When mixed with water, phospholipids form droplet spheres with a double-layered phospholipid bilayer. The hydrophilic heads facing the water and the hydrophobic tails facing each other. This traps a compartment of water in the middle separated from the external water by the hydrophobic sphere. This naturally-occurring structure is called a liposome, and is similar to a membrane surrounding a cell (see pxx).
There are 20 different R groups, and so 20 different amino acids. Since each R group is slightly different, each amino acid has different properties, and this in turn means that proteins can have a wide range of properties. The table on the next page shows the 20 different R groups, grouped by property, which gives an idea of the range of properties. You do not need to learn these, but it is interesting to see the different structures, and you should be familiar with the amino acid names. You may already have heard of some, such as the food additive monosodium glutamate, which is simply the sodium salt of the amino acid glutamate. There are 3-letter and 1-letter abbreviations for each amino acid.
Polypeptides Amino acids are joined together by peptide bonds. The reaction involves the formation of a molecule of water in another condensation polymerisation reaction:
When two amino acids join together a dipeptide is formed. Three amino acids form a tripeptide. Many amino acids form a polypeptide. e.g.:
In a polypeptide there is always one end with a free amino (NH 2 ) group, called the N-terminus, and one end with a free carboxyl (COOH) group, called the C-terminus.
In a protein the polypeptide chain may be many hundreds of amino acids long. Amino acid polymerisation to form polypeptides is part of protein synthesis. It takes place in ribosomes, and is special because it requires an RNA template. The sequence of amino acids in a polypeptide chain is determined by the sequence of the bases in DNA. Protein synthesis is studied in detail on pxx.
The Twenty Amino Acid R-Groups Simple R groups Basic R groups
Glycine Gly G
Lysine Lys K
Alanine Ala A
Arginine Arg R
Valine Val V
Histidine His H
Leucine Leu L
Asparagine Asn N
Isoleucine Ile I
Glutamine Gln Q
Hydroxyl R groups Acidic R groups
Serine Ser S
Aspartate Asp D
Threonine Thr T
Glutamate Glu E
Sulphur R groups Ringed R groups
Cysteine Cys C
Phenylalanine Phe F
Methionine Met M
Tyrosine Tyr Y
Cyclic R group Tryptophan Proline Pro P Trp W
3. Tertiary Structure This is the complete structure formed by the folding up of a polypeptide chain. Every protein has a unique tertiary structure, which is responsible for its properties and function. For example the shape of the active site in an enzyme is due to its tertiary structure. The tertiary structure is held together by bonds between the R groups of the amino acids in the protein, and so depends on what the sequence of amino acids is. These bonds include:
hydrogen bonds, which are weak but numerous. Ionic bonds (or salt bridges) between oppositely-charged R-groups e.g. in lysine or arginine with
than covalent bonds. covalent S–S bonds called sulphur bridges between two cysteine amino acids, which are much stronger.
4. Quaternary Structure Almost all working proteins are actually composed of more than one polypeptide chain, and the quaternary structure is the arrangement of the different chains. There are a huge variety of quaternary structures e.g.:
Haemoglobin consists of four chains arranged in a tetrahedral (pyramid) structure.
Antibodies comprise four chains arranged in a Y-shape.
The enzyme ATP synthase is composed of 22 chains forming a rotating motor.
Collagen consists of three chains in a triple helix structure.
Actin consists of hundreds of globular chains arranged in a long double helix.
These four structures are not real stages in the formation of a protein, but are simply a convenient classification that scientists invented to help them to understand proteins. In fact proteins fold into all these structures at the same time, as they are synthesised.
-S-
-S- -S-
-S-
The final three-dimensional shape of a protein can be classified as globular or fibrous.
Globular Proteins e.g. Haemoglobin The vast majority of proteins are globular, i.e. they have a compact, roughly spherical structure. This group includes enzymes, membrane proteins, receptors, transport proteins and storage proteins.
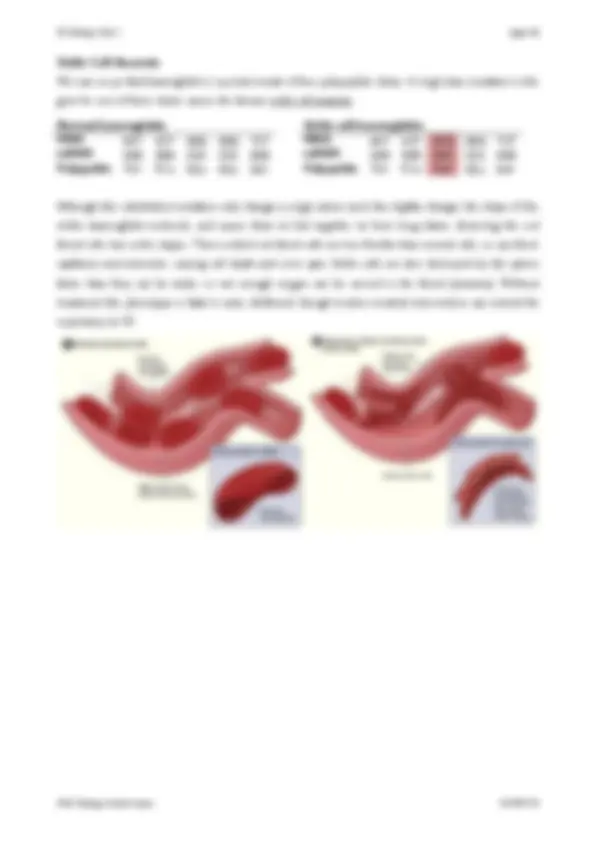
Haemoglobin is a globular protein found in red blood cells. One molecule is composed of four globular polypeptide chains called globins. There are two chains with 141 amino acids each and two chains with 146 amino acids each, giving a total of 574 amino acids. Each chain contains a small non-polypeptide group called haem, which has an iron atom at its centre. The haem groups are attached to the globin polypeptide chains by covalent sulphur bridges.
One oxygen molecule (O 2 ) can bind to each iron atom, so a haemoglobin molecule can bind up to four O 2 molecules. The polypeptide chains provide a suitable environment for the oxygen molecules to bind reversibly, so haemoglobin acts as an effective oxygen transport protein.
Fibrous Proteins e.g. Collagen Fibrous proteins are long and thin, like ropes. They tend to have structural roles, such as collagen (bone), keratin (hair), tubulin (cytoskeleton), actin (muscle), fibrin (blood clots) and fibroin (silk). They are always composed of many polypeptide chains.
Collagen is a fibrous protein found in bone and cartilage. A single molecule consists of three long polypeptide chains linked by numerous hydrogen bonds and wrapped round each other in a triple helix. Each polypeptide chain is about 10,000 amino acids long and contains a simple repeating sequence of just three amino acids (e.g. Gly-Pro-Ala). Many collagen molecules bind together to form a fibril, and many of these fibrils link to form fibres. These collagen fibres have high tensile strength and give strength and flexibility to cartilage, ligaments, tendons, bone, skin and blood vessels. Collagen is also found in the cornea and lens of the eye. The synthesis of collagen requires vitamin C, which is why vitamin C deficiency causes the disease scurvy, where connective tissue breaks down.