







































Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
These are the Lecture Slides of Material Science for Engineers which includes Structure of Wood, Moisture Content, Density of Wood, Mechanical Properties of Wood, Expansion and Contraction of Wood, Concrete Materials, Properties of Concrete etc. Key important points are: v
Typology: Slides
1 / 53

This page cannot be seen from the preview
Don't miss anything!














































Chapter 22 – Corrosion and Wear
Section 22.
Chemical Corrosion
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning
is a trademark used herein under license.™
Figure 22.1 Molten lead is held in thick steel pots during refining. In this case, the molten lead has attacked a weld in a steel plate and cracks have developed. Eventually, the cracks propagate through the steel, and molten lead leaks from the pot.
Electrochemical corrosion - Corrosion produced by the development of a current in an electrochemical cell that removes ions from the material.
Electrochemical cell - A cell in which electrons and ions can flow by separate paths between two materials, producing a current which, in turn, leads to corrosion or plating.
Oxidation reaction - The anode reaction by which electrons are given up to the electrochemical cell.
Reduction reaction - The cathode reaction by which electrons are accepted from the electrochemical cell.
Section 22.
Electrochemical Corrosion
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning™ is a trademark used herein under license.
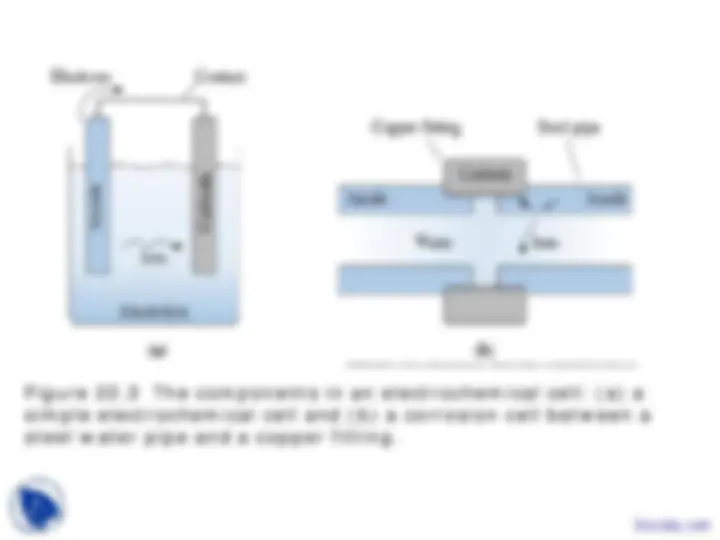
Figure 22.3 The components in an electrochemical cell: (a) a simple electrochemical cell and (b) a corrosion cell between a steel water pipe and a copper fitting.
Electrode potential - Related to the tendency of a material to corrode. The potential is the voltage produced between the material and a standard electrode.
emf series - The arrangement of elements according to their electrode potential, or their tendency to corrode.
Nernst equation - The relationship that describes the effect of electrolyte concentration on the electrode potential in an electrochemical cell.
Faraday’s equation - The relationship that describes the rate at which corrosion or plating occurs in an electrochemical cell.
Section 22.
The Electrode Potential in
Electrochemical Cells
©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning
is a trademark used herein under license.™
Figure 22.5 The half-cell used to measured the electrode potential of copper under standard conditions. The electrode potential of copper is the potential difference between it and the standard hydrogen electrode in an open circuit. Since E 0 is great than zero, copper is cathodic compared with the hydrogen electrode.
Design a process to electroplate a 0.1-cm-thick layer of copper onto a 1 cm × 1 cm cathode surface.
Example 22.2 SOLUTION
In order for us to produce a 0.1-cm-thick layer on a 1 cm 2 surface area, the weight of copper must be:
Example 22. Design of a Copper Plating Process
From Faraday’s equation, where M Cu = 63:54 g/mol and n = 2:
Example 22.2 SOLUTION
Therefore, we might use several different combinations of current and time to produce the copper plate:
Our choice of the exact combination of current and time might be made on the basis of the rate of production and quality of the copper plate. A current of ~ 1 A and a time of ~ 45 minutes are not uncommon in electroplating operations.
Example 22.3 SOLUTION
Suppose that in a corrosion cell composed of copper and zinc, the current density at the copper cathode is 0.05 A/cm 2. The area of both the copper and zinc electrodes is 100 cm^2. Calculate (1) the corrosion current, (2) the current density at the zinc anode, and (3) the zinc loss per hour.
Example 22.4 SOLUTION
Example 22. Copper-Zinc Corrosion Cell
Polarization - Changing the voltage between the anode and cathode to reduce the rate of corrosion.
Section 22.
The Corrosion Current and
Polarization
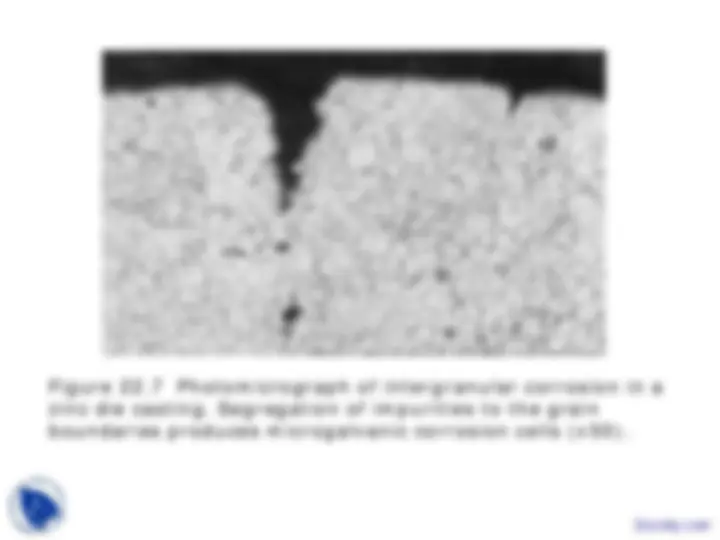
Intergranular corrosion - Corrosion at grain boundaries because grain boundary segregation or precipitation produces local galvanic cells.
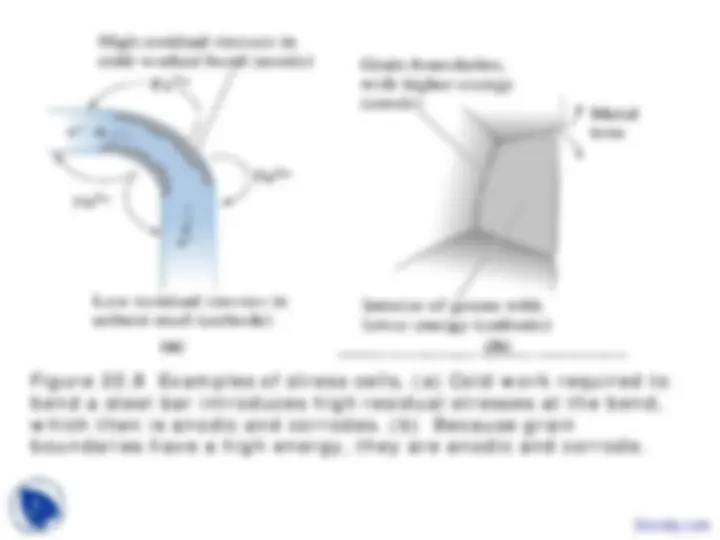
Stress corrosion - Deterioration of a material in which an applied stress accelerates the rate of corrosion.
Oxygen starvation - In the concentration cell, low- oxygen regions of the electrolyte cause the underlying material to behave as the anode and to corrode.
Crevice corrosion - A special concentration cell in which corrosion occurs in crevices because of the low concentration of oxygen.
Section 22.
Types of Electrochemical Corrosion