
















Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Classification of dispersed systems & their general characteristics, size & shapes of colloidal particles, classification of colloids & comparative account of their general properties. Optical, kinetic & electrical properties. Effect of electrolytes, coacervation, peptization& protective action
Typology: Assignments
1 / 23

This page cannot be seen from the preview
Don't miss anything!
















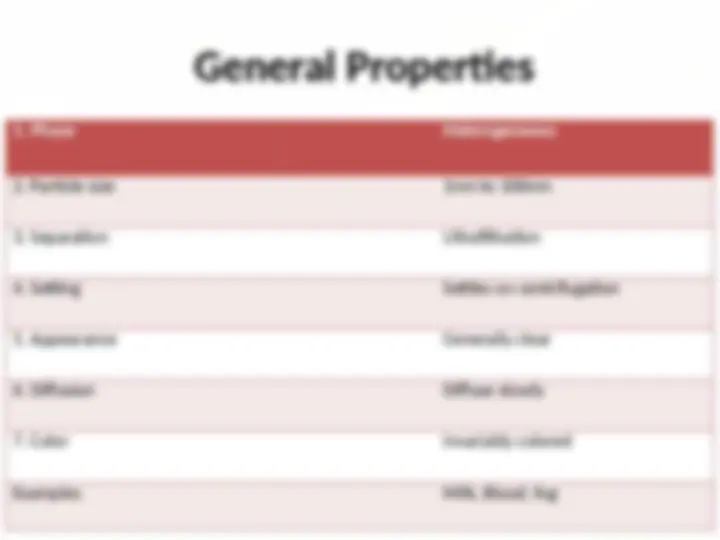
A mixture in which very small particles of one substance are
distributed evenly throughout another substance.
The word “colloid” was derived from the Greek, “kolla” for
glue.
Colloid is short synonym for colloidal system.
The heterogeneous biphasic system.
Size of colloidal particle ranges from 1nm to 100nm.
There two criteria's for classification of
are
colloids.
**1. Based on the state of Aggregation of phase
On the basis of affinity between two
phases, colloidal solutions are classified into
sols Examples
sols of Metals,
sulphur
2. Lyophilic sols
Examples gums,
used for
the
Two methods are generally
preparation of colloidal
solutions.
**1. By Condensation
Brownian motion
Rapid, random, zigzag movement of colloidal
particles through dispersion medium.
This movement is due to bombardment of
colloidal particles by the molecules of
dispersion medium.
Tyndall Effect
Light scattering by colloidal particles.
Bright cone of scattered light is called Tyndall
cone.
The colloidal solutions prepared by any
method contain different impurities.
They can be separated by
**1. Dialysis
Therapy
Colloidal system are used as therapeutic
agents in different areas.
Colloidal medicines are more effective and are
easily absorbed in our system.
Example ,penicillin and streptomycin.
It is used as disinfectants.
It is a homeopathic
remedy used to treat
skin conditions such as
psoriasis, eczema etc.
It is used in treatment of
cancer.
It is used as a remedy for
burns, arthritis, parasites,
viral and bacterial infections.
It is an unrivalled anti-biotic.
It destroy all types of virus, bad fungi and
bacteria.
It promotes healing.
Increase solubility, stability and taste of
compounds
Used as additives in drugs
E.g. Quaternary Ammonium salts